Calpain1 inhibition enhances autophagy-lysosomal pathway and ameliorates tubulointerstitial fibrosis in Nephronophthisis
- PMID: 40319239
- PMCID: PMC12049798
- DOI: 10.1186/s10020-025-01231-4
Calpain1 inhibition enhances autophagy-lysosomal pathway and ameliorates tubulointerstitial fibrosis in Nephronophthisis
Abstract
Background: Nephronophthisis (NPH) is classified under the category of renal ciliopathies and is the most common genetic disease leading to renal failure in children. Early-onset and progressive renal tubulointerstitial fibrosis represents one of the most significant features, culminating in renal insufficiency. However, the molecular mechanism of tubulointerstitial fibrosis remains unclear. Previously, we constructed an NPH mouse model via CRISPR-Cas9. This mouse model demonstrated typical features of tubulointerstitial fibrosis. In this study, we aimed to explore the pathogenesis of tubulointerstitial fibrosis in NPH and identify early intervention targets in both the NPH models and patients.
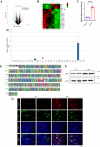
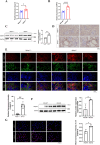
Methods: In this study, transcriptome changes in mouse kidneys were analyzed through RNA sequencing to explore the molecular mechanisms of renal tubulointerstitial fibrosis in NPH. We found an increased abundance of calpain1 in both the NPH models and patients. Pathway enrichment analysis indicated autophagy-lysosomal pathway was altered in the NPH models. Western blot, immunofluorescence or immunohistochemical staining were used to verify the expression of calpain1. We also detected autophagy activities in NPH models by lysotracker staining and transmission electron microscopy (TEM). Epithelial or mesenchymal-specific markers and Masson's trichrome staining were used to detect the status of tubulointerstitial fibrosis. Furthermore, NPH models were treated with a calpain1 inhibitor to explore the role of calpain1 in autophagy-lysosomal pathway and tubulointerstitial fibrosis.
Results: The increased abundance of calpain1 impaired the autophagy-lysosomal pathway and induced tubulointerstitial fibrosis by promoting epithelial-to-mesenchymal transition. On the other hand, calpain1 inhibition could enhance the autophagy-lysosomal pathway and ameliorate the phenotypes of tubulointerstitial fibrosis in NPH models.
Conclusions: Calpain1-mediated autophagy-lysosomal pathway disorder may be an important cause of tubulointerstitial fibrosis in NPH. Calpain1 may have therapeutic implications for renal tubulointerstitial fibrosis.
Keywords: Calpain1; Inherited kidney disease; Nephronophthisis; Renal ciliopathies; Renal tubulointerstitial fibrosis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Research involving human data was performed in accordance with the Declaration of Helsinki of 1975, as revised in 1983. All recruited subjects agreed to participate in the experiment and signed the consent form. Research involving human data was approved by Guangdong Provincial People's Hospital (Ethics Approval Number: S2023-010–01). All animals were maintained according to the ARRIVE1 guidelines, and all protocols for animal use and euthanasia were reviewed and approved by the Institutional Animal Care and Use Committee of Nanfang Hospital, Southern Medical University (Ethics Approval Number: IACUC-LAC-20220723–001). Consent for publication: Consent for publication was obtained from the patients mentioned in this study. Copies of consent forms could be sent upon request. Competing interests: The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
- Grant No.2023119212359160/Medical Scientific Research Foundation of Guangdong Province of China
- 2025A04J4728/Guangzhou Municipal Science and Technology Project
- Grant No. 81670610/National Natural Science Foundations of China
- Grant No. 2020A1515010286 and 2022A1515012307/Guangdong Natural Science Foundation
LinkOut - more resources
Full Text Sources
Medical

