Lymph node macrophage-targeted interferon alpha boosts anticancer immune responses by regulating CD169-positive phenotype of macrophages
- PMID: 40319320
- PMCID: PMC12049019
- DOI: 10.1186/s12943-025-02324-8
Lymph node macrophage-targeted interferon alpha boosts anticancer immune responses by regulating CD169-positive phenotype of macrophages
Abstract
Background: CD169+ macrophages in lymph nodes (LNs) activate cytotoxic T lymphocytes (CTLs), which play a crucial role in anticancer immunity, through antigen presentation and co-stimulation by CD169. Interferon alpha (IFNα) is capable of inducing the CD169+ phenotype of macrophages; however, its clinical applications have been hindered by pharmacokinetic limitations-low LN distribution and an inability to target macrophages. To overcome these issues, this study genetically fused mouse IFNα (mIFNα) with mannosylated mouse serum albumin (Man-MSA), and investigated the antitumor effects of the hybrid protein (Man-MSA-mIFNα) or its add-on effects with programmed death-ligand 1 (PD-L1) blockade.
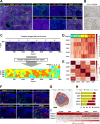
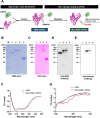
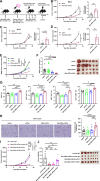
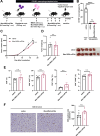
Methods: To confirm the possibility of CD169+ macrophage-mediated T cell priming, positional information about individual immune cells in LNs of cancer patients was obtained. Traits of Man-MSA-mIFNα, which was prepared using Pichia pastoris to form the high-mannose structure, were characterized by several physicochemical methods. To evaluate the lymphatic drainage of Man-MSA-mIFNα, radioiodine or Cy5-labeled Man-MSA-mIFNα was subcutaneously administered in mice, and then the radioactivity or fluorescence in LNs was analyzed. CD169-diphtheria toxin (DT) receptor (CD169-DTR) mice in which LN CD169+ macrophages can be depleted by DT injection were used to verify whether the antitumor effect of Man-MSA-mIFNα is dependent on LN CD169+ macrophages.
Results: Multiplex tissue imaging predicted close proximity of CD169+ macrophages and T cells and positive correlation between the number of CD169+ macrophages and T cells in neighborhoods in LNs of cancer patients. Physicochemical analyses showed that Man-MSA-mIFNα was formed from the fusion of the intact Man-MSA and mIFNα. Man-MSA-mIFNα efficiently induced the CD169+ phenotype of macrophages by its high LN distribution and macrophage-targeting capability, and significantly exerted antitumor activity through CD8+ T cell activation in the LNs, whereas its antitumor effects were canceled in CD169-DTR mice. Finally, combination therapy with PD-L1 blockade markedly suppressed tumor growth in MB49-bearing mice, which exhibit resistance to PD-L1 blockade monotherapy.
Conclusions: The present study successfully designed and developed Man-MSA-mIFNα, which efficiently induces the CD169+ phenotype in LN macrophages, contributing to the antitumor immunity. The findings suggest that our novel strategy targeting CD169⁺ macrophages could be a promising immunotherapy for cancer patients who are unresponsive to immune checkpoint inhibitors.
Keywords: Albumin; CD169; Cancer immunotherapy; Drug delivery system; IFNα; Lymph node; Macrophage; Targeted therapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study design was approved by the Institutional Review Board of Kumamoto University (#1169) in accordance with the guidelines for Good Clinical Practice and the Declaration of Helsinki. Paraffin-embedded LN samples were prepared from specimens obtained from patients diagnosed with colorectal cancer that were surgically resected at Izumi General Hospital (Izumi, Kagoshima, Japan). Written informed consent was obtained from all patients, and the study design was approved by the review board (#57). The need for individual patient consent for inclusion in the study was waived by the Institutional Review Board of Kumamoto University (#1169). All animal experiments were conducted using procedures approved by the experimental animal ethics committee at Kumamoto University (A2021-021). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

