Safety and efficacy with esketamine in treatment-resistant depression: long-term extension study
- PMID: 40319349
- PMCID: PMC12143125
- DOI: 10.1093/ijnp/pyaf027
Safety and efficacy with esketamine in treatment-resistant depression: long-term extension study
Abstract
Importance: The rates of relapse and suicide risk are higher in treatment-resistant depression (TRD) vs non-treatment-resistant major depressive disorder. Even among patients with TRD who initially respond, the majority (70%) relapse within 6 months.
Objective: To evaluate the long-term safety and efficacy of esketamine nasal spray, combined with an oral antidepressant, in patients with TRD.
Design: Phase 3, open-label, single-arm long-term extension study (SUSTAIN-3) conducted from June 2016 to December 2022.
Setting: Outpatient.
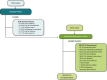
Participants: Adults with TRD who participated in ≥1 of 6 phase 3 "parent" studies continued esketamine by either entering a 4-week induction phase followed by an optimization/maintenance phase of variable duration (n = 458) or directly entering the optimization/maintenance phase of SUSTAIN-3 (n = 690), based on their individual response to study drug at the endpoint of the parent study.
Interventions: Intranasal esketamine dosing was flexible, twice-weekly during induction and individualized to depression severity during optimization/maintenance (weekly, every-other-week, or every-4-weeks), under direct supervision by site staff.
Main outcomes and measures: To assess the long-term safety of esketamine. Efficacy endpoints included the change in depressive symptoms, assessed by the Montgomery-Åsberg Depression Rating Scale (MADRS).
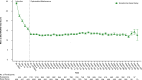
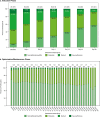
Results: A total of 1148 patients were enrolled. Total exposure to esketamine was 3777 cumulative patient-years. Mean (median, range) exposure to esketamine in SUSTAIN-3 was 42.9 (45.8, range 0-79) months. The most common adverse events were headache (36.9%), dizziness (33.9%), nausea (33.6%), dissociation (25.5%), nasopharyngitis (23.8%), somnolence (23.1%), dysgeusia (20.2%), and back pain (20.0%). During the study, 5.3% and 6.4% of participants discontinued due to lack of efficacy or adverse event, respectively. Nine participants died: COVID-19-related (n = 3), pneumonia (n = 2), and completed suicide, myocardial infarction, multiple injuries, unknown cause (n = 1 each). The mean MADRS total score decreased during induction, and this reduction persisted during optimization/maintenance (mean [SD] change from baseline-to-phase endpoint of each phase: induction: -12.8 [9.73]; optimization/maintenance: + 0.2 [9.93]). A total of 35.6% of participants were in remission at the induction endpoint, and 48.5% and 49.6% at week 112 and optimization/maintenance endpoint, respectively.
Conclusions and relevance: In the SUSTAIN-3 final dataset, no new safety signals were identified during long-term treatment with intermittently-dosed esketamine, combined with oral antidepressant, and improvement in depression generally persisted among participants who remained on maintenance treatment. These results add to the accumulated evidence on TRD treatment with esketamine.
Trial registration: clinicaltrials.gov identifier: NCT02782104.
Keywords: efficacy; esketamine; long-term; safety; treatment-resistant depression.
© The Author(s) 2025. Published by Oxford University Press on behalf of CINP.
Conflict of interest statement
N.Z., L.(N.)C., R.L., T.D., W.C.D, V.P., and D.-J.F are employees of Johnson & Johnson, Titusville NJ, United States (N.Z., L.(N.)C., R.L., T.D.,D.-J.F), San Diego, CA, United States (W.C.D), and Beerse, Belgium (V.P.), as was R.L.M. at the time this study was being conducted (now retired), and all are stockholders of Johnson & Johnson.
In the past year G.S. has served as a consultant to Actinogen Medical, Alto Neuroscience, ATAI, Axsome Therapeutics, Biogen, Biohaven Pharmaceuticals, Boehringer Ingelheim International GmbH, Bristol-Myers Squibb, Clexio, Daiichi Sankyo, Denovo, Douglas Pharmaceuticals, Biopharma, Douglas Pharmaceuticals, EMA Wellness, Embark, Freedom Biosciences, Gilgamesh, Holmusk, Merck, Neumora, Neurocrine, Newleos, Novartis, Otsuka, Perception Neuroscience, Relmada Therapeutics, Sage Pharmaceuticals, Seaport Pharmaceuticals, Seelos Pharmaceuticals, Supernus, Taisho Pharmaceuticals, Tetricus, Transcend Therapeutics, Usona Institute, and XW Labs; and received research contracts from Merck over the past 12 months. G.S. holds equity in Biohaven Pharmaceuticals, Freedom Biosciences, Gilead, Relmada, and Tetricus. He is a co-inventor on a US patents (#8,778,979) and (#12090145) held by Yale University. Yale University, but not G.S., has a financial relationship with Janssen Pharmaceuticals and may receive financial benefits from this relationship.
S.T.W. has received contract funding for clinical trials from Sage Therapeutics, Oui Therapeutics, and Janssen (administered through Yale University). He has received consulting fees from Sage Therapeutics, Oui Therapeutics, and Janssen.
A.H.Y. has received compensation for lectures and advisory board participation from Allegan, AstraZeneca, Bionomics, Boehringer Ingelheim, COMPASS, Eli Lilly, Janssen, LivaNova, Lundbeck, Neurocentrx, Novartis, Sage Pharmaceuticals, Servier, Sumitomo Dainippon Pharma, and Sunovion. He has received grant funding (past and present) from: NIMH (USA); CIHR (Canada); NARSAD (USA); Stanley Medical Research Institute (USA); MRC (UK); Wellcome Trust (UK); Royal College of Physicians (Edin); BMA (UK); UBC-VGH Foundation (Canada); WEDC (Canada); CCS Depression Research Fund (Canada); MSFHR (Canada); NIHR (UK); Janssen (UK), EU Horizon 2020, Maudsley Biomedical Research Centre at South London, and Maudsley NHS Foundation Trust and King’s College London.
A.L.T.L. has provided consulting services to Daiichi Sankyo, Pfizer, Sanofi-Aventis, Lundbeck, Apsen, Libbs, EMS, Biogen, Cristalia, Janssen, and LivaNova. He has received funds for contracted research from Boehringer Ingelheim, Eli Lilly, Biophytis, Genentech, Novo Nordisk, Celltrion, Novartis, EOM, Parexel, Genova, Cellavita, Janssen Pharmaceuticals, Azidus, IQVIA, Acadia Pharmaceuticals, Neumora Therapeutics, and PPD.
J.-W.P. has received compensation for lectures and advisory board participation from AstraZeneca, Janssen, Dongwha Pharm, Pfizer Korea, and Korea Otsuka Pharm. He has received grant funding (past and present) from: NHIDI (South Korea); NECA (South Korea); Ministry of Health and Welfare (South Korea); Janssen (Korea), Bukwang Pharma (South Korea); and Ministry of Science and ICT (South Korea).
Figures
References
-
- American Psychiatric Association. Practice Guideline for the Treatment of Patients with Major Depressive Disorder. 3rd ed. American Psychiatric Association; 2013. Accessed December 12, 2024. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/...
-
- Dold M, Bartova L, Fugger G, et al. Major depression and the degree of suicidality: results of the European group for the study of resistant depression (GSRD). Int J Neuropsychopharmacol. 2018; 21:539–549. https://doi.org/ 10.1093/ijnp/pyy009 - DOI - PMC - PubMed
-
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006; 163:1905–1917. https://doi.org/ 10.1176/ajp.2006.163.11.1905 - DOI - PubMed
-
- Rush AJ. STAR*D: what have we learned? Am J Psychiatry. 2007; 164:201–204. https://doi.org/ 10.1176/ajp.2007.164.2.201 - DOI - PubMed
-
- United States Food & Drug Administration. FDA news release 2019. Silver Spring, MD. Accessed December 12, 2024. https://www.fda.gov/news-events/press-announcements/fda-approves-new-nas...
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

