A Prospective Evaluation of a Three-Gene Host Response Signature to Classify Tuberculosis Severity in Children
- PMID: 40319382
- PMCID: PMC12117649
- DOI: 10.1093/jpids/piaf041
A Prospective Evaluation of a Three-Gene Host Response Signature to Classify Tuberculosis Severity in Children
Abstract
Background: Children with non-severe TB may benefit from short-course treatment, but point-of-care tools are needed to stratify disease severity. We prospectively evaluated the Cepheid Xpert MTB-Host Response (HR) prototype cartridge for distinguishing TB severity in children with pulmonary TB (PTB) in The Gambia and Uganda.
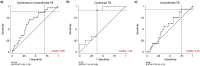
Methods: We included children <15 with microbiologically confirmed or clinically diagnosed unconfirmed PTB. Severity was defined using the World Health Organization (WHO) guidelines for a four-month, drug-susceptible regimen. Capillary or venous blood was tested with the HR cartridge for PCR-based detection of 3 mRNA genes and calculation of a TB score from cycle thresholds. We generated receiver operating characteristic curves with the TB score to classify severe TB and assessed if Xpert-HR could achieve the WHO target accuracy for treatment optimization (≥90% sensitivity, ≥70% specificity).
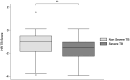
Results: Among 106 children, the median age was 4 years (IQR 1-7), 56.6% were female, and 13.2% were living with HIV. In all children with PTB, Xpert-HR achieved an AUC of 0.67 (95% CI 0.55-0.78), with 89.3% sensitivity (95% CI 71.8-97.7) and 29.5% specificity (95% CI 19.7-40.9, cutoff ≤ -0.60). By confirmation status, Xpert-HR approached the target accuracy in children with Confirmed TB, with 62.5% specificity (95% CI 24.5-91.5) at 91.7% sensitivity (95% CI 61.5-99.8, cut-off ≤ -1.349). Among children with Unconfirmed TB, specificity was lower (24.3%, 95% CI 14.8-36.0) at 93.8% sensitivity (95% CI 69.8-99.8, cutoff ≤ -0.450). Target accuracy was almost achieved in children 5-9 regardless of confirmation status (100% sensitivity [95% CI 71.5-100], 66.7% specificity [95% CI 43.0-85.4], cutoff ≤ -1.35), but specificity (28.2%, 95% CI 18.6-39.5) was lower for children < 5 (92.9% sensitivity, 95% CI 76.5-99.1, cutoff ≤ -0.550).
Conclusions: Xpert-HR approached the target accuracy to stratify PTB severity in older children and those with Confirmed TB but had lower specificity in children with Unconfirmed TB. Child-specific signatures may be needed to improve performance in younger children with paucibacillary disease.
Keywords: child; gene signature; host-response; severity; tuberculosis.
© The Author(s) 2025. Published by Oxford University Press on behalf of The Journal of the Pediatric Infectious Diseases Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- World Health Organization. Global tuberculosis report 2024; 2024:68.
-
- Marais BJ, Gie RP, Schaaf HS, et al.The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis. 2004;8:392–402. - PubMed
-
- Shingadia D, Novelli V.. Diagnosis and treatment of tuberculosis in children. Lancet Infect Dis. 2003;3:624–632. https://doi.org/10.1016/s1473-3099(03)00771-0 - DOI - PubMed
-
- Li Y, Zhu Y, Zhong Q, Zhang X, Shu M, Wan C.. Serious adverse reactions from anti-tuberculosis drugs among 599 children hospitalized for tuberculosis. Pediatr Infect Dis J. 2017;36:720–725. https://doi.org/10.1097/INF.0000000000001532 - DOI - PubMed
-
- World Health Organization. WHO operational handbook on tuberculosis. In: Module 5: Management of Tuberculosis in Children and Adolescents. World Health Organization; 2022. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

