Clinical Trial: Effect of a 28-Day Low FODMAP Diet on Gastrointestinal Symptoms Associated With Endometriosis (EndoFOD)-A Randomised, Controlled Crossover Feeding Study
- PMID: 40319391
- PMCID: PMC12107219
- DOI: 10.1111/apt.70161
Clinical Trial: Effect of a 28-Day Low FODMAP Diet on Gastrointestinal Symptoms Associated With Endometriosis (EndoFOD)-A Randomised, Controlled Crossover Feeding Study
Abstract
Background: Gastrointestinal symptoms affect most women with endometriosis, but therapeutic interventions are poorly defined.
Aims: To compare the effects of a 28-day low fermentable oligo-, di- and mono-saccharides and polyols (FODMAP) or control diet on gastrointestinal symptom severity in individuals with endometriosis and poorly controlled gastrointestinal symptoms.
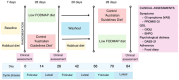
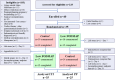
Method: In this single-blinded randomised, controlled cross-over feeding trial, we randomised women aged ≥ 18 years to 28 days of either a low FODMAP (< 5 g/day FODMAPs) or control diet (20 g/day FODMAPs), both modelled on Australian Dietary Guidelines, before a ≥ 28-day washout and crossover to the alternate diet. The primary outcome was the proportion of responders defined according to the response in overall gastrointestinal symptoms on a 100-mm visual analogue scale. Secondary outcomes included gastrointestinal symptoms, quality of life and psychological status.
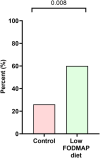
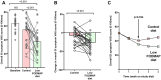
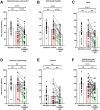
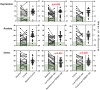
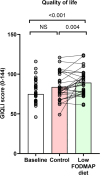
Results: Of 35 women randomised (mean age 31; 95% confidence interval 29, 33 years), 21 (60%) responded to the low FODMAP diet compared with 9 (26%) to the control diet (p = 0.008). In the 4th week of the dietary intervention, overall symptom scores were 35 (21, 42) mm on the low FODMAP diet and 58 (55, 65) mm on the control diet (p < 0.001). Abdominal pain, bloating, stool form and quality of life for both gastrointestinal and endometriosis-associated scales were significantly better for the low FODMAP diet compared with the control diet, but not overall for perceived stress, anxiety or depression.
Conclusions: The low FODMAP diet ameliorates gastrointestinal symptoms related to endometriosis and improves quality of life. Confirmation of these findings in a real-world setting is required.
Trial registration: The trial is registered with the Australian New Zealand Clinical Trials Registry (ACTRN12621000153819).
Keywords: diet; endometriosis; gastroenterology; nutrition; quality of life.
© 2025 The Author(s). Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
J.G.M., J.E.V. and P.R.G. work in a department that financially benefits from the sales of a digital application (Monash University FODMAP diet app), patient booklets, cookbooks and online courses, all of which relate to the low FODMAP diet therapy. J.G.M. and J.E.V.'s salaries are paid by the above‐stated FODMAP commercial activities. P.R.G. and R.E.B. are consultants or advisory board members for Anatara and Atmo Biosciences and have received speaker honoraria from Dr Falk Pharma. R.E.B. has also received speaker honoraria from Bayer. P.R.G. is also a consultant or advisory board member for Topas and Comvita. P.R.G. has received research grants for investigator‐driven studies and/or speaker honoraria from Atmo Biosciences and Mindset Health. P.R.G. and D.S. are shareholders in Atmo Biosciences. D.S. has received consulting and speaking honoraria from Procter & Gamble and is an employee of Atmo Biosciences. Research funding for this project came from a competitively awarded grant from the Australian Government's Medical Research Future Fund. The funder had no role in study design, data collection, analysis, interpretation or report writing. No other relationships or activities could appear to have influenced the submitted work.
Figures

References
-
- Horne A. W. and Missmer S. A., “Pathophysiology, Diagnosis, and Management of Endometriosis,” British Medical Journal 379 (2022): e070750. - PubMed
-
- Australian Institute of Health and Welfare , “Endometriosis, How Common is Endometriosis?” (2023), https://www.aihw.gov.au/reports/chronic‐disease/endometriosis‐in‐austral....
-
- Deepak Kumar K., Appleby‐Gunnill B., and Maslin K., “Nutritional Practices and Dietetic Provision in the Endometriosis Population, With a Focus on Functional Gut Symptoms,” Journal of Human Nutrition and Dietetics 36, no. 4 (2023): 1529–1538. - PubMed
-
- Maroun P., Cooper M. J., Reid G. D., and Keirse M. J., “Relevance of Gastrointestinal Symptoms in Endometriosis,” Australian & New Zealand Journal of Obstetrics & Gynaecology 49, no. 4 (2009): 411–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
