Effect of hydrogen sulfide on alpha-synuclein aggregation and cell viability
- PMID: 40320462
- PMCID: PMC12050307
- DOI: 10.1038/s41598-025-99794-z
Effect of hydrogen sulfide on alpha-synuclein aggregation and cell viability
Abstract
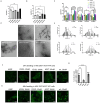
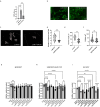
Parkinson's disease (PD) is a progressive neurodegenerative movement disorder characterized by nigrostriatal degeneration and aggregation of α-synuclein (α-Syn) with accumulation of insoluble aggregates in Lewy bodies. Familial mutations in α-Syn are associated with the development of PD. Accumulation of insoluble aggregates results in neuronal toxicity. Identification of compounds that inhibit seeding activity of α-Syn is of great importance. Here we investigate the potential of H2S donor, sodium hydrosulfide (NaHS), to inhibit α-Syn aggregation. We examined the effect of NaHS on fibril growth kinetics and the structural change of α-Syn fibrils formed by self-seeding and cross-seeding of wild-type (wt) and PD familial α-Syn mutations. NaHS slowed both self- and cross-seeded A53T α-Syn fibril formation but not wild-type fibril formation. We observed a decrease in the formed fibril length in vitro. We examined the effect on fibril formation within cells. NaHS significantly reduced the number and filament length of formed oligomers in an α-Syn overexpressing cell model. Furthermore, NaHS rescued viability of A53T α-Syn overexpressing cells seeded with wt- and mutant preformed fibrils. These results support a conformation-specific effect of hydrogen sulfide on alpha-synuclein aggregation and cell viability which deserves further exploration for therapeutic potential.
Keywords: Hydrogen sulfide; Parkinson disease; Protein aggregation; α-Synuclein.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

