In vitro models of microglia: a comparative study
- PMID: 40320508
- PMCID: PMC12050316
- DOI: 10.1038/s41598-025-99867-z
In vitro models of microglia: a comparative study
Abstract
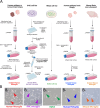
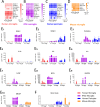
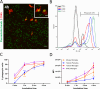
Microglia perform key homeostatic functions to protect the central nervous system (CNS). However, in many brain disorders their protective functions are abrogated, contributing to disease progression. Therefore, studies of microglial function are critical to developing treatments for brain disorders. Different in vitro microglia models have been established, including primary human and rodent cells, induced pluripotent stem cell (iPSC)-derived models, and immortalised cell lines. However, a direct comparative analysis of the phenotypic and functional characteristics of these models has not been undertaken. Accurate modelling of human microglia in vitro is critical for ensuring the translatability of results from the bench to the brain. Therefore, our study aimed to characterise and compare commonly utilised in vitro microglia models. We assessed four established microglia models: primary human microglia, human iPSC-derived microglia, the human microglial clone 3 (HMC3) cell line, and primary mouse microglia, with primary human brain pericytes acting as a negative control. Primary human microglia, iPSC-derived microglia, and mouse microglia stained positive for myeloid-cell markers (Iba1, CD45 and PU.1), while HMC3 cells only stained positive for mural-cell markers (PDGFRβ and NG2). Distinct secretomes were observed in all cell models in response to inflammatory treatment, with iPSC-derived microglia showing the most significant inflammatory secretions. Notably, nitric oxide was only secreted by mouse microglia. Although all cell types exhibited phagocytic capacity, primary human microglia and iPSC-derived microglia displayed significantly higher levels of phagocytosis. Overall, comparative analysis revealed notable differences between human microglia, iPSC-derived microglia, HMC3 cells and mouse microglia. Such differences should be considered when using these models to study human brain diseases. Experimental findings obtained from mouse models or cell lines should ultimately be cross validated to ensure the translatability of results to the human condition.
Keywords: In vitro models; Comparative analysis; Human microglial clone 3 (HMC3); Microglia; iPSC-derived microglia.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Studies undertaken using human tissue were approved by the Northern Regional Ethics Committee (New Zealand), with informed consent obtained from all donors. All methods were carried out in accordance with approved guidelines. Competing interests: The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

