Has FDA's Drug Development Tools Qualification Program Improved Drug Development?
- PMID: 40320513
- PMCID: PMC12181102
- DOI: 10.1007/s43441-025-00790-2
Has FDA's Drug Development Tools Qualification Program Improved Drug Development?
Abstract
Background: The Drug Development Tools (DDTs) Qualification program creates a pathway to evaluate Clinical Outcome Assessments (COAs) that capture a specific concept of interest (COI) in a specified Context of Use (COU). If successfully qualified, a COA can be relied upon to measure a COI that has an application in drug development and regulatory decision-making. Thus, qualified COAs are important DDTs. This analysis aims to assess the Food and Drug Administration's (FDA's) performance reviewing applications in the COA Qualification Program, as well as the uptake of qualified COAs in drug development to date.
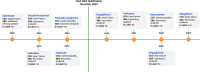
Methods: In order to assess the use of qualified COAs in drug development, we analyzed the Summary Basis of Approvals (SBA) retrieved from Drugs@FDA and the COA compendium. The submission and review dates for the Letter of Intent (LOI), Qualification Plan (QP), and Full Qualification Package (FQP) steps were retrieved from Center for Drug Evaluation and Research (CDER) & Center for Biologics Evaluation and Research's (CBER) database, as well as the FDA COA Qualification Program website.
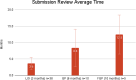
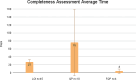
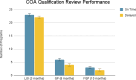
Results: Our analysis showed that 86 COAs were listed on the FDA COA website, with a majority of them being Patient Reported Outcomes (PRO). Completeness Assessment (CA) for each portion of submission, as well as review times for the LOI, QP, and FQP steps vary widely, with 46.7% of submissions having a review time exceeding the published targets. To date, 7 COAs (8.1%) have achieved qualification, and one (1.1%) has been denied after undergoing all steps for qualification. On average, it takes 6 years for a COA to be qualified. Our analysis of FDA's approval documents shows that the Agency has relied on qualified COAs to support benefit-risk assessment of 11 medicines. Only three of the seven qualified COAs have been used to support benefit-risk assessment of medicines. The three qualified COAs that have been used are KCCQ, E-RS, and EXACT. Each of these has been used to support multiple indication claims. KCCQ - cardiomyopathy for 2 medicines, heart failure for 6 medicines; E-RS - chronic obstructive pulmonary disease (COPD) for 1 medicine; and EXACT - COPD for 3 medicines. Note: E-RS and EXACT were both used in aclidinium bromide/formoterol/fumarate. In each case they were used as secondary or exploratory endpoints, none as primary endpoints. Only 1 qualified COA was included in drug labels.
Conclusion: The lengthy and unpredictable nature of the COA Qualification Program review timelines poses a risk for tool developers and sponsors intending to qualify a new COA, to use an existing COA or sponsors intending to qualify and use a new COA in the drug development process. Our findings show that, to date, the DDT Qualification Program has not significantly improved the inclusion of qualified COAs in clinical development plans to support regulatory decision-making and label claims, and therefore the impact of the pathway to facilitate the use of innovative tools has been limited. To improve the utility of this program, FDA should publicly share the timelines so participants can be better prepared to integrate into their development programs. Furthermore, FDA should clearly articulate how and when COAs can be used in drug development.
Keywords: COA; Context of use; FDA; Primary/secondary endpoints; Qualification.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing Interests: This research was conducted as part of Felix Yang’s Howard University Pharmaceutical Industry Fellowship Program in partnership with Genentech Inc, A Member of the Roche Group. Imein Bousnina, Anne Madej, and Rasika Kalamegham are full time employees of Genentech, A Member of the Roche Group. None of the authors have any financial interest other than employment by Genentech.
Figures

References
-
- U.S. Food and Drug Administration. Qualification of the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score and its Component Scores A Patient-Reported Outcome Instrument for Use in Clinical Investigations in Heart Failure. 2020.
-
- U.S. Food and drug administration. qualification statement qualification of Non-Small cell lung Cancer symptom assessment questionnaire (NSCLC-SAQ): A Patient-Reported outcome instrument. 2018.
-
- U.S. Food and Drug Administration. Attachment - Guidance on Qualification Process for Drug Development Tools: Qualification of Exacerbations of Chronic Pulmonary Disease Tool for Measurement of Symptoms of Acute Bacterial Exacerbation of Chronic Bronchitis in Patients With Chronic Obstructive Pulmonary Disease. 2014. FDA-2013-D-1630.
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

