Determinants of physical activity level in pediatric oncological patients treated with cardiotoxic therapy - a study protocol
- PMID: 40320516
- PMCID: PMC12051312
- DOI: 10.1186/s12887-025-05714-5
Determinants of physical activity level in pediatric oncological patients treated with cardiotoxic therapy - a study protocol
Abstract
Background: Childhood cancer and its therapy, especially that involving potentially cardiotoxic cancer treatment, can structurally affect muscle strength and heart function, and therefore may result in lowered physical activity (PA). This study protocol aims to find the significant determinants of PA levels in pediatric oncological patients 1-5 years after heart-toxic chemotherapy and/or radiation of the heart region.
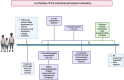
Methods: The study group will include children 1-5 years after completing cancer therapy involving cardiotoxicity risk factors. The primary outcome of interest is the PA measured with an ActiGraph GT3X Accelerometer for 14 consecutive days and the assessment of WHO pediatric age-adjusted PA norms achievement. Assessed PA levels will be evaluated according to the possible determinants of PA: disease/treatment related risk factors, treatment complications, possible complications after the treatment, cardiac function with echocardiography including 2D and 3D strain imaging, physical function and muscle strength in ALPHA (Assessing Levels of Physical Activity) health-related fitness test battery and exercise capacity in cardiopulmonary exercise testing. The self-efficacy and motivation to PA, quality of life (QoL), lifestyle, socio-demographic, and anthropogenic factors as well as knowledge about the positive impact of PA will be evaluated with original and validated questionnaires. The PA determinants of the study group will be compared to the results of the control group of children in the same follow-up period (> 1 year < 5 years) after completing cancer therapy without cardiotoxic methods.
Discussion: The results may contribute to the development of future recommendations on prophylactic and therapeutic approaches, as well as proper lifestyle recommendations for children in long-term follow-up after cardiotoxic cancer therapy. The determinants will be used to develop targeted exercise prescriptions and exercise programs.
Trial registration: NCT06256068.
Keywords: Cardiotoxicity; Childhood cancer; Exercise capacity; Pediatric oncology; Physical activity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study will be conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving patients are approved by the local Ethic Examining Committee of Human Research of the Medical University of Warsaw (Decision of Approval: KB/54/A2023). Informed consent will be obtained from all subjects involved in the study. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Swerdlow SHCE, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J. WHO classification of tumours of haematopoietic and lymphoid tissues. WHO classification of tumours. Revised 4th Edition, Volume 2. Lyon: International Agency for Research on Cancer (IARC); 2017.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical