The PLUS study: efficacy of triclosan coated suture (VicrylPlus®) to reduce infection in primary suture of childbirth related perineal tears - a randomized controlled trial
- PMID: 40320545
- PMCID: PMC12051262
- DOI: 10.1186/s40748-025-00211-0
The PLUS study: efficacy of triclosan coated suture (VicrylPlus®) to reduce infection in primary suture of childbirth related perineal tears - a randomized controlled trial
Abstract
Background: Preventing infection in primary sutured perineal tears after childbirth is crucial to avoid systemic antibiotic use and potential complications from poor healing. This study aimed to investigate the efficacy of an antibacterial, triclosan-coated suture (VicrylPlus®) in reducing infection in primary sutured childbirth-related perineal tears.
Methods: The PLUS study was a single-center, single-blinded, adaptive parallel-group randomized trial conducted at Lund University Hospital, Sweden. Women aged ≥ 18 years with a perineal tear at childbirth were randomly assigned in a 1:1 ratio to either the control group (conventional-absorbable suture, Vicryl®) or the intervention group (triclosan-coated- absorbable suture, VicrylPlus®).
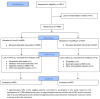
Results: Out of 1921 eligible women, 1890 were randomized to either Vicryl® (n = 953) or VicrylPlus® (n = 937). There were no significant demographic differences between the groups. The most common type of tear in both groups was a second-degree tear (Vicryl® 66.2% (n = 625), VicrylPlus® 67.5% (n = 625)). Encompassing all types of deeper tears in the analysis there was a significantly decrease in infection after suturing with VicrylPlus® 4% (n = 28) versus Vicryl® 6.8% (n = 47); (OR 0.57, 95% CI 0.35-0.91, P = 0.024). When analyzing different tears separately, there was a non-significant increase in infection for first-degree tears with VicrylPlus® 0.8% (n = 2) versus Vicryl® 3.9% (n = 8); (OR 4.75, 95% CI 1.00-22.63, P = 0.050). However, for second-degree tears, the infection rate was significantly reduced with VicrylPlus® 4.4% (n = 27) versus Vicryl® 7.2% (n = 44); (OR 0.63, 95% CI 0.36-0.98, P = 0.05) and for third-degree and unclassified tears there was a non-significant decrease in infections with VicrylPlus® 5.3% (n = 1) versus Vicryl® 14.3% (n = 2); (OR 0.33, 95% CI 0.03-4.10, P = 0.561), respectively, VicrylPlus® 0% versus Vicryl® 1.7% (n = 1); (OR 0.98, 95% CI 0.95-1.02, P = 0.462).
Conclusion: The use of triclosan coated sutures significantly reduces the risk of infection in primary sutured childbirth-related perineal tears by 43%, except for first-degree tears. Further research is needed to determine whether their effectiveness remains consistent across the other specific types of deeper tears in a larger study population.
Trial registration: ClinicalTrials (NCT02863874), posted 11/08/2016, retrospectively registered. Approved by the regional ethical committee before start of enrollment (Dnr 2015/774).
Keywords: Antibacterial sutures; Childbirth; Episiotomies; Perineal tears; Surgical site infection.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: The study was approved by the regional ethical committee (Dnr 2015/774). Informed consent was obtained after childbirth, following the ethical committee’s decision. This decision was based on the necessity for women to receive treatment irrespective of study participation, as both types of sutures were part of the standard assortment in the birthing unit. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Frohlich J, Kettle C. Perineal care. BMJ Clin Evid [Internet]. 2015 [cited 2022 Jun 16];2015. Available from: www.clinicalevidence.com. - PMC - PubMed
-
- Schmidt PC, Fenner DE. Repair of episiotomy and obstetrical perineal lacerations (first to fourth). Am J Obstet Gynecol [Internet]. 2022 [cited 2023 Jul 11]; Available from: 10.1016/j.ajog.2022.07.005 - PubMed
-
- Kettle C, Tohill S. Perineal care. BMJ Clin Evid [Internet]. 2011 [cited 2016 May 17];2011. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21481287
-
- Wiseman O, Rafferty AM, Stockley J, Murrells T, Bick D. Infection and wound breakdown in spontaneous second-degree perineal tears: An exploratory mixed methods study. Birth [Internet]. 2019 Mar 1 [cited 2019 Mar 9];46(1):80–9. Available from: 10.1111/birt.12389 - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical