Luminal Phospholipase D Attacks Bacterial Membranes in Dictyostelium discoideum Phagosomes
- PMID: 40320828
- PMCID: PMC12242099
- DOI: 10.1111/mmi.15367
Luminal Phospholipase D Attacks Bacterial Membranes in Dictyostelium discoideum Phagosomes
Abstract
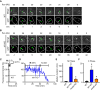
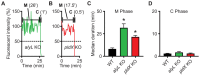
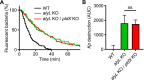
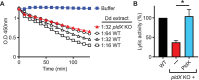
Phagocytic cells ingest bacteria and kill them in phagosomes. A variety of molecular mechanisms allow the killing and destruction of bacteria in phagosomes, but their complete list and relative importance remain poorly defined. Here we have used Dictyostelium discoideum amoebae as model phagocytic cells. Our results reveal that PldX, a luminal phospholipase D, plays an important role in the phagosomal destruction of ingested bacteria. Analysis of bacterial destruction in wild-type and pldX KO living cells suggests that PldX participates in the permeabilization of the bacterial membrane. The bacteriolytic activity of D. discoideum extracts was also measured in vitro: extracts from pldX KO cells exhibit significantly less bacteriolytic activity than wild-type cells, confirming the role of PldX in the lysis of bacterial membranes. These results identify luminal phospholipase D as a major player in the permeabilization of bacterial membranes in phagosomes.
Keywords: Dictyostelium discoideum; bacterial lysis; intracellular destruction; permeabilization of bacterial membrane; phospholipase D.
© 2025 The Author(s). Molecular Microbiology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts interest.
Figures
References
-
- Alibaud, L. , Cosson P., and Benghezal M.. 2003. “ Dictyostelium discoideum Transformation by Oscillating Electric Field Electroporation.” BioTechniques 35: 78–83. - PubMed
-
- Ayadi, I. , Lamrabet O., Munoz‐Ruiz R., Jauslin T., Guilhen C., and Cosson P.. 2024. “Extracellular and Intracellular Destruction of Pseudomonas aeruginosa by Dictyostelium discoideum Phagocytes Mobilize Different Antibacterial Mechanisms.” Molecular Microbiology 121: 69–84. - PubMed
-
- Bozzaro, S. 2013. “The Model Organism Dictyostelium discoideum .” In Methods in Molecular Biology, vol. 983, 17–37. Humana Press. - PubMed
-
- Choi, S. Y. , Huang P., Jenkins G. M., Chan D. C., Schiller J., and Frohman M. A.. 2006. “A Common Lipid Links Mfn‐Mediated Mitochondrial Fusion and SNARE‐Regulated Exocytosis.” Nature Cell Biology 8: 1255–1262. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

