Novel Oxadiazole-Based Bioisostere of Caffeic Acid Phenethyl Ester: Synthesis, Anticancer Activity, and Inhibition of Lipoxygenase Product Biosynthesis
- PMID: 40320854
- PMCID: PMC12050906
- DOI: 10.1002/ddr.70099
Novel Oxadiazole-Based Bioisostere of Caffeic Acid Phenethyl Ester: Synthesis, Anticancer Activity, and Inhibition of Lipoxygenase Product Biosynthesis
Abstract
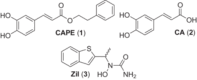
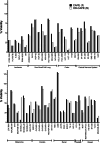
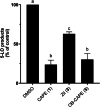
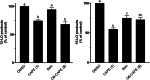
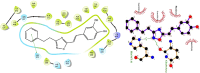
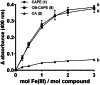
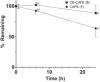
Caffeic acid phenethyl ester (1), a honeybee propolis component, possesses many bioactive properties, making it a useful scaffold for drug research. Further, CAPE (1) is a more effective inhibitor of the biosynthesis of 5-lipoxygenase (5-LO) products compared to Zileuton, the only clinically-approved direct 5-LO inhibitor. However, CAPE (1) suffers from a poor metabolic profile, being rapidly metabolized to caffeic acid (CA). In this study, we synthesized and performed several biological assays on a new bioisostere of CAPE (1) possessing a 1,2,4-oxadiazole ring. The new bioisostere (OB-CAPE (5)) has a similar antiproliferative effect to CAPE (1) on NCI-60 cancer cell lines and maintains the activity of CAPE (1) as an inhibitor of the biosynthesis of 5-, 12- and 15-LO products and as an iron chelator. In human polymorphonuclear leukocytes, OB-CAPE (5) inhibits the biosynthesis of 5-LO products with an IC50 of 0.93 µM compared to 1.0 µM for CAPE (1). Both compounds have similar antioxidant activity, with IC50 values of 1.2 µM for OB-CAPE (5) and 1.1 µM for CAPE (1). The new hydrogen bond predicted for the oxadiazole ring and the GLN363 amino acid in the 5-LO active site may explain the small improvement in the affinity of OB-CAPE (5) for the protein compared to CAPE (1). Finally, stability studies in human plasma reveal that OB-CAPE (5) is 25% more stable than CAPE (1). Therefore, the increase in stability associated with the replacement of the ester function with its bioisostere, while maintaining the anti-inflammatory and anticancer properties of CAPE (1), suggests that OB-CAPE (5) may be a comparable yet more stable candidate for in vivo studies in disease models.
Keywords: anti‐leukotriene therapy; bioisosteres; caffeic acid phenethyl ester; inflammation.
© 2025 The Author(s). Drug Development Research published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
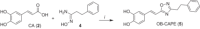
Similar articles
-
Caffeic acid phenethyl ester and its amide analogue are potent inhibitors of leukotriene biosynthesis in human polymorphonuclear leukocytes.PLoS One. 2012;7(2):e31833. doi: 10.1371/journal.pone.0031833. Epub 2012 Feb 9. PLoS One. 2012. PMID: 22347509 Free PMC article.
-
Structure-activity relationship of caffeic acid phenethyl ester analogs as new 5-lipoxygenase inhibitors.Chem Biol Drug Des. 2017 Apr;89(4):514-528. doi: 10.1111/cbdd.12874. Epub 2016 Nov 15. Chem Biol Drug Des. 2017. PMID: 27717142
-
Synthesis of bioisosteres of caffeic acid phenethyl ester: 1,3,4-oxadiazole derivatives containing a catechol fragment with anti-inflammatory activities in vitro and in vivo.Bioorg Chem. 2025 Feb;155:108123. doi: 10.1016/j.bioorg.2025.108123. Epub 2025 Jan 3. Bioorg Chem. 2025. PMID: 39756202
-
In vivo and in vitro antıneoplastic actions of caffeic acid phenethyl ester (CAPE): therapeutic perspectives.Nutr Cancer. 2013;65(4):515-26. doi: 10.1080/01635581.2013.776693. Nutr Cancer. 2013. PMID: 23659443 Review.
-
Radio-Modulatory Potential of Caffeic Acid Phenethyl Ester: A Therapeutic Perspective.Anticancer Agents Med Chem. 2018;18(4):468-475. doi: 10.2174/1871520617666171113143945. Anticancer Agents Med Chem. 2018. PMID: 29141565 Review.
References
-
- Allain, E. P. , Boudreau L. H., Flamand N., and Surette M. E.. 2015. “The Intracellular Localisation and Phosphorylation Profile of the Human 5‐Lipoxygenase Δ13 Isoform Differs From That of Its Full Length Counterpart.” PLoS One 10, no. 7: e0132607. 10.1371/journal.pone.0132607. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

