Mitochondria and Endoplasmic Reticulum Contact Site as a Regulator of Proteostatic Stress Responses in Neurodegenerative Diseases
- PMID: 40320859
- PMCID: PMC12183774
- DOI: 10.1002/bies.70016
Mitochondria and Endoplasmic Reticulum Contact Site as a Regulator of Proteostatic Stress Responses in Neurodegenerative Diseases
Abstract
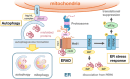
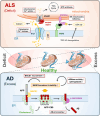
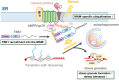
Recent evidence indicates that the mitochondria-endoplasmic reticulum (ER) contact site is a novel microdomain essential for cellular homeostasis. Various proteins are accumulated at the mitochondria-associated membrane (MAM), an ER subcomponent closely associated with the mitochondria, contributing to Ca2+ transfer to the mitochondria, lipid synthesis, mitochondrial fission/fusion, and autophagy. These functions are disrupted in the diseases, particularly in neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS) and Alzheimer's disease. In this review, we summarize the disruption of protein homeostasis in various neurodegenerative diseases, present recent works on the mechanisms of MAM aberration, including ours mainly focused on ALS, and then discuss challenges and prospects for future MAM-targeted therapies in neurodegenerative diseases.
Keywords: mitochondria‐associated membranes; neurodegenerative diseases; protein homeostasis.
© 2025 The Author(s). BioEssays published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Markovinovic A., Greig J., Martín‐Guerrero S. M., Salam S., and Paillusson S., “Endoplasmic Reticulum‐Mitochondria Signaling in Neurons and Neurodegenerative Diseases,” Journal of Cell Science 135 (2022): jcs248534. - PubMed
-
- Barazzuol L., Giamogante F., and Calì T., “Mitochondria Associated Membranes (MAMs): Architecture and Physiopathological Role,” Cell Calcium 94 (2021): 102343. - PubMed
-
- Vance J. E., “Phospholipid Synthesis in a Membrane Fraction Associated With Mitochondria,” Journal of Biological Chemistry 265 (1990): 7248–7256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 23K06826/Ministry of Education, Culture, Sports, Science and Technology, Japan/Japan Society for the Promotion of Science
- 19KK0214/Ministry of Education, Culture, Sports, Science and Technology, Japan/Japan Society for the Promotion of Science
- 22H00467/Ministry of Education, Culture, Sports, Science and Technology, Japan/Japan Society for the Promotion of Science
- JP22ek0109426/Japan Agency for Medical Research and Development
- JP24wm0425014/Japan Agency for Medical Research and Development
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

