Pathogenic TNNT1 variants are associated with aberrant thin filament compliance and myofibre hyper-contractility
- PMID: 40320982
- PMCID: PMC12206437
- DOI: 10.1113/JP288109
Pathogenic TNNT1 variants are associated with aberrant thin filament compliance and myofibre hyper-contractility
Abstract
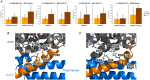
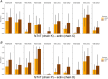
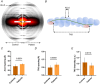
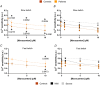
In skeletal muscle, troponin T (TnT) exists in two isoforms, slow skeletal TnT (ssTnT) and fast skeletal TnT (fsTnT), encoded by the TNNT1 and TNNT3 genes, respectively. Nonsense or missense TNNT1 variants have been associated with skeletal muscle weakness and contractures and a histopathological appearance of nemaline myopathy (NM) on muscle biopsy. Little is known about how TNNT1 mutations ultimately lead to muscle dysfunction, preventing the development of targeted therapeutic interventions. Here, we aimed to identify the underlying molecular biophysical mechanisms, by investigating isolated skeletal myofibres from patients with TNNT1-related NM as well as from controls through a combination of structural and functional assays. Our studies revealed variable and unusual ssTnT and fsTnT expression patterns and post-translational modifications. We also observed that, in the presence of TNNT1 variants, the thin filament was more compliant, and this was associated with a higher myofibre Ca2+ sensitivity. Altogether, our findings suggest TnT remodelling as the key mechanism ultimately leading to molecular and cellular hyper-contractility, and then inhibitors of altered contractility as potential therapeutic modalities for TNNT1-associated NM. KEY POINTS: No therapeutic treatment exists for patients with genetic TNNT1 mutations and skeletal muscle weakness/contractures. In these patients, expression and post-translational modifications of troponin T are severely disrupted. These are associated with changes in thin filament compliance where troponin T is located. All these induce muscle fibre hyper-contractility that can be reversed by mavacamten, a myosin ATPase inhibitor.
Keywords: congenital myopathy; contracture; force; skeletal muscle; troponin.
© 2025 The Author(s). The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
C.T.A.L. is an employee of Novo Nordisk A/S. This position began after their work on this paper and the position had no influence on the results or conclusions drawn. M.W.L. is the CEO and founder of Diverge Translational Science Laboratory, which works with numerous companies in the areas of therapeutic development and testing. His work at Diverge is unrelated to the therapeutic options discussed here and his position had no influence on the results or conclusions drawn. A.L.H. is an owner of Accelerated Muscle Biotechnologies Consultants LLC, which performed the X‐ray data reduction and analysis, but services rendered were not linked to outcome or interpretation.
Figures





References
-
- Abdulhaq, U. N. , Daana, M. , Dor, T. , Fellig, Y. , Eylon, S. , Schuelke, M. , Shaag, A. , Elpeleg, O. , & Edvardson, S. (2016). Nemaline body myopathy caused by a novel mutation in troponin T1 (TNNT1). Muscle, & Nerve, 53(4), 564–569. - PubMed
-
- Berg, S. , Kutra, D. , Kroeger, T. , Straehle, C. N. , Kausler, B. X. , Haubold, C. , Schiegg, M. , Ales, J. , Beier, T. , Rudy, M. , Eren, K. , Cervantes, J. I. , Xu, B. , Beuttenmueller, F. , Wolny, A. , Zhang, C. , Koethe, U. , Hamprecht, F. A. , & Kreshuk, A. (2019). ilastik: Interactive machine learning for (bio)image analysis. Nature Methods, 16(12), 1226–1232. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
