Tirzepatide 10 and 15 mg versus semaglutide 2.4 mg in people with obesity or overweight with type 2 diabetes: An indirect treatment comparison
- PMID: 40321113
- PMCID: PMC12146454
- DOI: 10.1111/dom.16401
Tirzepatide 10 and 15 mg versus semaglutide 2.4 mg in people with obesity or overweight with type 2 diabetes: An indirect treatment comparison
Abstract
Aims: Tirzepatide and semaglutide demonstrated clinically meaningful weight reduction in people with obesity or overweight and type 2 diabetes (T2D) in SURMOUNT-2 and STEP 2 clinical trials, respectively. In the absence of head-to-head trials, this study compared the efficacy of tirzepatide 10 and 15 mg with semaglutide 2.4 mg using an indirect treatment comparison.
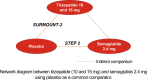
Materials and methods: Mean percent change in weight from baseline, weight reduction ≥5% and mean change in glycated haemoglobin (HbA1c [%]) were compared between tirzepatide 10/15 mg (week 72, SURMOUNT-2) and semaglutide 2.4 mg (week 68, STEP 2) applying the Bucher method to the efficacy estimand. Sensitivity analyses included the use of matching-adjusted indirect comparison, treatment regimen estimand and comparing study outcomes at 68 weeks.
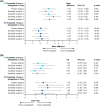
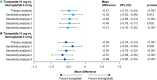
Results: Tirzepatide 10 and 15 mg were associated with significantly greater mean percent weight reductions versus semaglutide (mean difference, 10 mg: 2.57%; 15 mg: 4.79%, p < 0.01). Tirzepatide 15 mg had significantly higher odds of achieving ≥5% weight reduction (odds ratio 15 mg: 1.76, 95% CI 1.04-2.97, p = 0.035; odds ratio 10 mg: 1.24, 95% CI 0.75-2.04, p = 0.407), and both tirzepatide doses were associated with significantly greater reductions in HbA1c (%) levels (mean difference, 10 mg: 0.47%; 15 mg: 0.56%, p < 0.001) than semaglutide. Sensitivity analyses were generally consistent with the primary analysis, exceptions including when power was reduced in the matching-adjusted indirect comparison analyses and in the categorical weight reduction outcome.
Conclusions: This analysis suggested greater reductions in bodyweight and HbA1c (%) levels associated with tirzepatide 10 and 15 mg than with semaglutide 2.4 mg in people with obesity or overweight and T2D.
Keywords: indirect treatment comparison; obesity; semaglutide; tirzepatide; type 2 diabetes; weight reduction.
© 2025 Eli Lilly and Company. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
Emily R. Hankosky, Xuanyao He, Julia Fraseur Brumm, Fangyu Wang, Anthony Niemeyer and Xiaotian Michelle Zhang are employees and stockholders of Eli Lilly and Company, Indianapolis, United States. Raleigh Malik was an employee of Eli Lilly and Company at the time of the study. W. Timothy Garvey has served as a consultant on advisory boards for Boehringer Ingelheim, Eli Lilly and Company, Novo Nordisk, Pfizer, Fractyl Health, Alnylam Pharmaceuticals, Inogen, Zealand, Carmot/Roche, Regeneron and Merck, and as a site principal investigator for multi‐centred clinical trials sponsored by his university and funded by Novo Nordisk, Eli Lilly and Company, Epitomee, Neurovalens and Pfizer.
Figures
References
-
- Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288‐298. - PubMed
-
- NIDDK . Health risks of overweight & obesity . https://www.niddk.nih.gov/health‐information/weight‐management/adult‐ove...
-
- CDC . National Diabetes Statistics Report 2020. Estimates of diabetes and its burden in the United States . 2020. Accessed August 28, 2024. https://www.cdc.gov/diabetes/pdfs/data/statistics/national‐diabetes‐stat...
-
- Centers for Disease Control and Prevention (CDC) . Adult obesity facts . Accessed August 28, 2024. https://www.cdc.gov/obesity/adult‐obesity‐facts/index.html#:~:text=The%2...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

