High-throughput encapsulated nanodroplet screening for accelerated co-crystal discovery
- PMID: 40321178
- PMCID: PMC12044422
- DOI: 10.1039/d4sc07556k
High-throughput encapsulated nanodroplet screening for accelerated co-crystal discovery
Abstract
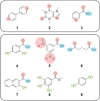
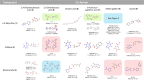
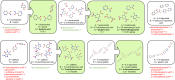
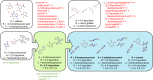
Co-crystals are composed of two or more chemically inequivalent molecular species, excluding solvents, generally in a stoichiometric ratio. Co-crystals are particularly important in pharmaceutical development, where a suitable co-crystal can significantly improve the physiochemical and pharmacokinetic properties of an active pharmaceutical ingredient. However, co-crystal discovery remains both practically challenging and resource intensive, requiring the extensive searching of complex experimental space. Herein, we demonstrate a high-throughput (HTP) nanoscale co-crystallisation method for the rapid screening of large areas of co-crystallisation space with minimal sample requirements, based on Encapsulated Nanodroplet Crystallisation (ENaCt). HTP co-crystallisation screening by ENaCt allowed rapid access to all 18 possible binary co-crystal combinations of 3 small molecules and 6 co-formers (A/B), through the use of 3456 individual experiments exploring solvent, encapsulating oil and stoichiometry, including 10 novel binary co-crystal structures elucidated by single crystal X-ray diffraction (SCXRD). Higher-order co-crystal (HOC) discovery, accessing co-crystals containing three or more molecules, is one of the most challenging co-crystal research areas, due to the highly complex experimental landscape that must be navigated. Herein, we further exemplify the power of ENaCt co-crystallisation by application to HOC discovery. HTP ENaCt co-crystallisation screening of three component (A/B/C) and four component (A/B/C/D) combinations gave ready access to both ternary and quaternary HOCs, each containing three or four different molecular species respectively. In total, 13 056 individual ENaCt experiments are presented resulting in 54 co-crystal structures by SCXRD, including 17 novel binary co-crystals, 8 novel ternary co-crystals and 4 novel quaternary co-crystals. ENaCt co-crystallisation is thus demonstrated to be a highly impactful and efficient tool in the search for small molecule co-crystals, through the employment of parallelised HTP nanoscale experimental workflows.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
JPM, MJH and MRP and authors of a patent application related to this work.
Figures




References
-
- Bond A. D. What Is a Co-Crystal? CrystEngComm. 2007;9:833–834. doi: 10.1039/B708112J. - DOI
- Aitipamula S. Banerjee R. Bansal A. K. Biradha K. Cheney M. L. Choudhury A. R. Desiraju G. R. Dikundwar A. G. Dubey R. Duggirala N. Ghogale P. P. Ghosh S. Goswami P. K. Goud N. R. Jetti R. R. K. R. Karpinski P. Kaushik P. Kumar D. Kumar V. Moulton B. Polymorphs, Salts, and Cocrystals: What's in a Name? Cryst. Growth Des. 2012;12:2147–2152. doi: 10.1021/cg3002948. - DOI
-
- Little M. A. Briggs M. E. Jones J. T. A. Schmidtmann M. Hasell T. Chong S. Y. Jelfs K. E. Chen L. Cooper A. I. Trapping Virtual Pores by Crystal Retro-Engineering. Nat. Chem. 2015;7:153–159. doi: 10.1038/nchem.2156. - DOI - PubMed
- Jiang H. Hu P. Ye J. Zhang K. K. Yi L. Hu W. Kloc C. Tuning of the Degree of Charge Transfer and the Electronic Properties in Organic Binary Compounds by Crystal Engineering: A Perspective. J. Mater. Chem. C. 2018;6:1884–1902. doi: 10.1039/C7TC04982J. - DOI
- Sun L. Wang Y. Yang F. Zhang X. Hu W. Cocrystal Engineering: A Collaborative Strategy toward Functional Materials. Adv. Mater. 2019;31:1902328. doi: 10.1002/adma.201902328. - DOI - PubMed
- Huang Y. Wang Z. Chen Z. Zhang Q. Organic Cocrystals: Beyond Electrical Conductivities and Field‐Effect Transistors (FETs) Angew. Chem., Int. Ed. 2019;58:9696–9711. doi: 10.1002/anie.201900501. - DOI - PubMed
- Liu Y. Li A. Xu S. Xu W. Liu Y. Tian W. Xu B. Reversible Luminescent Switching in an Organic Cocrystal: Multi‐Stimuli‐Induced Crystal‐To‐Crystal Phase Transformation. Angew. Chem., Int. Ed. 2020;59:15098–15103. doi: 10.1002/anie.202002220. - DOI - PubMed
- Li M. Hua B. Liang H. Liu J. Shao L. Huang F. Supramolecular Tessellations via Pillar[N]Arenes-Based Exo–Wall Interactions. J. Am. Chem. Soc. 2020;142:20892–20901. doi: 10.1021/jacs.0c11037. - DOI - PubMed
- Ning G.-H. Cui P. Sazanovich I. V. Pegg J. Zhu Q. Pang Z.-F. Wei R.-J. Towrie M. Jelfs K. E. Little M. A. Cooper A. I. Organic Cage Inclusion Crystals Exhibiting Guest-Enhanced Multiphoton Harvesting. Chem. 2021;7:3157–3170. doi: 10.1016/j.chempr.2021.09.016. - DOI
- Wang Y. Wu H. Jones L. O. Mosquera M. A. Stern C. L. Schatz G. C. Stoddart J. F. Color-Tunable Upconversion-Emission Switch Based on Cocrystal-To-Cocrystal Transformation. J. Am. Chem. Soc. 2023;145:1855–1865. doi: 10.1021/jacs.2c11425. - DOI - PubMed
-
- Delori A. Urquhart A. J. Oswald I. D. H. Supramolecular Hair Dyes: A New Application of Cocrystallization. CrystEngComm. 2016;18:5360–5364. doi: 10.1039/C6CE01001F. - DOI
- Bushuyev O. S. Friščić T. Barrett C. J. Controlling Dichroism of Molecular Crystals by Cocrystallization. Cryst. Growth Des. 2016;16:541–545. doi: 10.1021/acs.cgd.5b01361. - DOI
- Sangtani E. Mandal S. K. Sreelakshmi A. S. Munshi P. Gonnade R. G. Salts and Cocrystals of Furosemide with Pyridines: Differences in π-Stacking and Color Polymorphism. Cryst. Growth Des. 2017;17:3071–3087. doi: 10.1021/acs.cgd.6b01868. - DOI
- Li M. Li Z. Zhang Q. Peng B. Zhu B. Wang J. Liu L. Mei X. Fine-Tuning the Colors of Natural Pigment Emodin with Superior Stability through Cocrystal Engineering. Cryst. Growth Des. 2018;18:6123–6132. doi: 10.1021/acs.cgd.8b01002. - DOI
-
- Xiao Y. Wu C. Zhou L. Yin Q. Yang J. Cocrystal Engineering Strategy for Sustained Release and Leaching Reduction of Herbicides: A Case Study of Metamitron. Green Chem. 2022;24:8088–8099. doi: 10.1039/D2GC02949A. - DOI
- Xiao Y. Wu C. Cui P. Zhou L. Yin Q. Pursuing Green and Efficient Agriculture from Molecular Assembly: A Review of Solid-State Forms on Agrochemicals. J. Agric. Food Chem. 2023;71:10500–10524. doi: 10.1021/acs.jafc.3c01084. - DOI - PubMed
- Xiao Y. Wu C. Feng S. Chen K. Zhou L. Yin Q. Temperature-Responsive Cocrystal Engineering for Efficacious Delivery of Poorly Water-Soluble Herbicide. Cryst. Growth Des. 2023;23:8381–8395. doi: 10.1021/acs.cgd.3c01045. - DOI
-
- Bolton O. Matzger A. J. Improved Stability and Smart-Material Functionality Realized in an Energetic Cocrystal. Angew. Chem., Int. Ed. 2011;50:8960–8963. doi: 10.1002/anie.201104164. - DOI - PubMed
- Landenberger K. B. Bolton O. Matzger A. J. Energetic–Energetic Cocrystals of Diacetone Diperoxide (DADP): Dramatic and Divergent Sensitivity Modifications via Cocrystallization. J. Am. Chem. Soc. 2015;137:5074–5079. doi: 10.1021/jacs.5b00661. - DOI - PubMed
- Bellas M. K. Matzger A. J. Peroxosolvate Discovery Method Leads to First Cocrystal with Three Energetic Components. Chem. Commun. 2022;58:8806–8809. doi: 10.1039/D2CC02024F. - DOI - PubMed
LinkOut - more resources
Full Text Sources

