Access to spirocyclic vinyl sulfones via radical cyclization and functional group migration
- PMID: 40321185
- PMCID: PMC12044546
- DOI: 10.1039/d5sc02555a
Access to spirocyclic vinyl sulfones via radical cyclization and functional group migration
Abstract
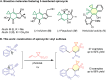
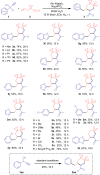
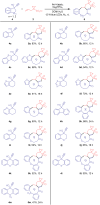
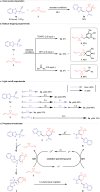
Spirocyclic vinyl sulfones, which incorporate the three-dimensional structure inherent to spiro compounds and the Michael acceptor reactivity associated with vinyl sulfones, hold promise for novel biological activities. The lack of efficient synthetic methods, however, hinders their extensive investigations in drug discovery and development. In this work, we describe a practical and versatile approach for the synthesis of multi-functionalized spirocyclic vinyl sulfones from easily available materials. The reaction proceeds efficiently through a cascade of radical cyclization followed by (hetero)aryl migration. The protocol features mild photocatalytic conditions and provides access to a diverse range of products, enabling the construction of complex scaffolds, including medium-sized ring-fused spirocyclic vinyl sulfones.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Wermuth C. G., in The Practice of Medicinal Chemistry, Elsevier Academic Press, San Diego, 2003
-
-
For selected reviews:
- Lin H. Danishefsky S. J. Angew. Chem., Int. Ed. 2003;42:36–51. doi: 10.1002/anie.200390048. - DOI - PubMed
- Galliford C. Scheidt K. Angew. Chem., Int. Ed. 2007;46:8748–8758. doi: 10.1002/anie.200701342. - DOI - PubMed
- Rios R. Chem. Soc. Rev. 2012;41:1060–1074. doi: 10.1039/C1CS15156H. - DOI - PubMed
- Undheim K. Synthesis. 2014;46:1957–2006. doi: 10.1055/s-0033-1338640. - DOI
- Smith L. K. Baxendale I. R. Org. Biomol. Chem. 2015;13:9907–9933. doi: 10.1039/C5OB01524C. - DOI - PubMed
- Xie X. Huang W. Peng C. Han B. Adv. Synth. Catal. 2018;360:194–228. doi: 10.1002/adsc.201700927. - DOI
- Xu P.-W. Yu J.-S. Chen C. Cao Z.-Y. Zhou F. Zhou J. ACS Catal. 2019;9:1820–1882. doi: 10.1021/acscatal.8b03694. - DOI
- Ramon R. T., Spiro Compounds: Synthesis and Applications, VCH, 2022, - DOI
- Kargbo R. B. ACS Med. Chem. Lett. 2025;16:216–218. doi: 10.1021/acsmedchemlett.5c00028. - DOI - PMC - PubMed
-
-
-
For selected reviews:
- Zheng Y. Tice C. M. Singh S. B. Bioorg. Med. Chem. Lett. 2014;24:3673–3682. doi: 10.1016/j.bmcl.2014.06.081. - DOI - PubMed
- Varela M. T. Dias G. G. de Oliveira L. F. N. de Oliveira R. G. Aguiar F. D. Nogueira J. P. Cruz L. R. Dias L. C. Eur. J. Med. Chem. 2025;287:117368. doi: 10.1016/j.ejmech.2025.117368. - DOI - PubMed
-
-
- Schobert R. Biersack B. Knauer S. Ocker M. Bioorg. Med. Chem. 2008;16:8592–8597. doi: 10.1016/j.bmc.2008.08.015. - DOI - PubMed
- Knauer S. Biersack B. Zoldakova M. Effenberger K. Milius W. Schobert R. Anti-Cancer Drugs. 2009;20:676–681. doi: 10.1097/CAD.0b013e32832e056a. - DOI - PubMed
- Schobert R. Seibt S. Mahal K. Ahmad A. Biersack B. Effenberger-Neidnicht K. Padhye S. Sarkar F. H. Mueller T. J. Med. Chem. 2011;54:6177–6182. doi: 10.1021/jm200359n. - DOI - PubMed
- Le P. Nodwell M. B. Eirich J. Sieber S. A. Chem.–Eur. J. 2019;25:12644–12651. doi: 10.1002/chem.201902919. - DOI - PMC - PubMed
- Linder B. Zoldakova M. Kornyei Z. Köhler L. H. F. Seibt S. Menger D. Wetzel A. Madarász E. Schobert R. Kögel D. Int. J. Mol. Sci. 2022;23:9056. doi: 10.3390/ijms23169056. - DOI - PMC - PubMed
-
- McMorris T. C. Anchel M. J. Am. Chem. Soc. 1965;87:1594–1600. doi: 10.1021/ja01085a031. - DOI - PubMed
- Kelner M. J. McMorris T. C. Beck W. T. Zamora J. M. Taetle R. Cancer Res. 1987;47:3186–3189. - PubMed
- Jaspers N. G. Raams A. Kelner M. J. Ng J. M. Yamashita Y. M. Takeda S. McMorris T. C. Hoeijmakers J. H. DNA Repair. 2002;1:1027–1038. doi: 10.1016/S1568-7864(02)00166-0. - DOI - PubMed
- Lehmann V. K. Huang A. Ibanez-Calero S. Wilson G. R. Rinehart K. L. J. Nat. Prod. 2003;66:1257–1258. doi: 10.1021/np030205w. - DOI - PubMed
- Schobert R. Knauer S. Seibt S. Biersack B. Curr. Med. Chem. 2011;18:790–807. doi: 10.2174/092986711794927766. - DOI - PubMed
- Glatt H. Pietsch K. E. Sturla S. J. Meinl W. Arch. Toxicol. 2014;88:161–169. doi: 10.1007/s00204-013-1097-2. - DOI - PubMed
- Uto Y. Sasaki K. Takahashi M. Morimoto K. Inoue K. Anal. Sci. 2019;35:789–792. doi: 10.2116/analsci.19P053. - DOI - PubMed
- Casimir L. Zimmer S. Racine-Brassard F. Jacques P.-É. Maréchal A. DNA Repair. 2023;122:103433. doi: 10.1016/j.dnarep.2022.103433. - DOI - PubMed
LinkOut - more resources
Full Text Sources

