This is a preprint.
Profiling the human luminal small intestinal microbiome using a novel ingestible medical device
- PMID: 40321269
- PMCID: PMC12047917
- DOI: 10.1101/2025.04.18.25326056
Profiling the human luminal small intestinal microbiome using a novel ingestible medical device
Abstract
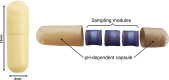
The invasive nature of sample collection for studying the small intestinal (SI) microbiome often results in its poor characterization. This study evaluated a novel ingestible medical device (MD) for SI luminal sample collection. A monocentric interventional trial (NCT05477069) was conducted on 15 healthy subjects. Metagenomics, metabolomics and culturomics assessed the MD's effectiveness in characterizing the healthy SI microbiome and identifying potential biomarkers. The SI microbiota differed significantly from the fecal microbiota, displaying high inter-individual variability, lower species richness, and reduced alpha diversity. A combined untargeted and semi-targeted LC-MS/MS metabolomics approach identified a distinct SI metabolic footprint, with bile acids and amino acids being the most abundant classes of metabolites. Host and host/microbe-derived bile acids were particularly abundant in SI samples. The application of a fast culturomics approach to two SI samples enabled species-level characterization, resulting in the identification of 90 bacterial species, including five potential novel species. The present study demonstrates the efficacy of our novel sampling MD in enabling comprehensive SI microbiome analysis through an integrative multi-omics approach, allowing the identification of distinct microbiome signatures between SI and fecal samples.
Keywords: Healthy subjects; Microbiome; Multi-omics; Sampling medical device; Small intestine.
Conflict of interest statement
DISCLOSURE STATEMENT A.T and T.S, as employee and CEO/co-founders of Pelican Health respectively, which markets intestinal sampling capsules, may face a conflict of interest due to their roles within the company. D.M, J.P.A and P.C are co-founder of Pelican Health. A.L.G is a co-founder for ALPIONER Therapeutics. P.C.D is an advisor and holds equity in Cybele, BileOmix and Sirenas and a Scientific co-founder, advisor and holds equity to Ometa, Enveda, and Arome with prior approval by UC-San Diego. P.C.D also consulted for DSM animal health in 2023. S.C.P, M.L and G.R are employees of Danone (France and Netherlands). All the other authors, declare that they have no conflict of interest.
Figures






References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
