Perspective on the use of fluorescence molecular imaging for peripheral and deep en face margin assessment
- PMID: 40321301
- PMCID: PMC12048172
- DOI: 10.1117/1.JBO.30.S1.S13711
Perspective on the use of fluorescence molecular imaging for peripheral and deep en face margin assessment
Abstract
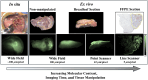
Significance: Current standard practice for margin assessment in solid tumor resection often leads to suboptimal results due to the inability to assess margins completely in a time-efficient manner. On the other hand, for small skin cancers, peripheral and deep en face margin assessment (PDEMA) offers 100% assessment of margins while sparing the utmost amount of normal surrounding tissues. Nonetheless, PDEMA is limited in its use owing to its lengthy tissue processing and imaging time as well as its requirement for high-quality frozen sections and real-time histologic analysis.
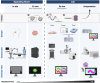
Aim: We aim to explore fluorescence molecular imaging (FMI) as a tool for resolving obstacles and integrating PDEMA into the surgeon-to-pathologist workflow for large solid tumors.
Approach: A review of recent pre-clinical and clinical studies using FMI to assess surgical margins was conducted to highlight promising fluorescence imaging technologies utilized in the surgical suite and laboratory.
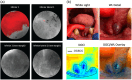
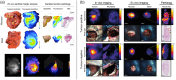
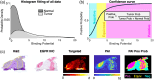
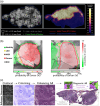
Results: FMI techniques that provide macroscopic resolution are efficient in time and have a notable ability to identify true negative tissue yet have limited capability in identifying true positive tissues. Moreover, meso- and microscopic FMI methods require additional time to attain a higher resolution but deliver an enhanced sensitivity in detecting true positive tissues. In both cases, experts are still required to learn to interpret the FMI signals, which prohibits a seamless clinical integration.
Conclusions: Our proposed margin assessment platform (MAP) incorporates both macroscopic and, meso- or microscopic imaging with post-processing and machine learning for interpretation, to enable the application of PDEMA into solid tumor surgery. MAP leverages the advantages of each technique and thoroughly tackles the limitations of time and expertise to optimize the efficiency and accuracy of margin assessment and ultimately improve clinical outcomes.
Keywords: fluorescence; molecular imaging; peripheral and deep en face margin assessment; tumor margin.
© 2025 The Authors.
Figures



Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Magnetic resonance perfusion for differentiating low-grade from high-grade gliomas at first presentation.Cochrane Database Syst Rev. 2018 Jan 22;1(1):CD011551. doi: 10.1002/14651858.CD011551.pub2. Cochrane Database Syst Rev. 2018. PMID: 29357120 Free PMC article.
-
A deep learning algorithm to detect cutaneous squamous cell carcinoma on frozen sections in Mohs micrographic surgery: a retrospective assessment.medRxiv [Preprint]. 2023 May 16:2023.05.14.23289960. doi: 10.1101/2023.05.14.23289960. medRxiv. 2023. Update in: Exp Dermatol. 2024 Jan;33(1):e14949. doi: 10.1111/exd.14949. PMID: 37293008 Free PMC article. Updated. Preprint.
-
Automated devices for identifying peripheral arterial disease in people with leg ulceration: an evidence synthesis and cost-effectiveness analysis.Health Technol Assess. 2024 Aug;28(37):1-158. doi: 10.3310/TWCG3912. Health Technol Assess. 2024. PMID: 39186036 Free PMC article.
-
Indocyanine Green Fluorescence Imaging for Colorectal Surgery: A Health Technology Assessment.Ont Health Technol Assess Ser. 2025 Jul 10;25(3):1-124. eCollection 2025. Ont Health Technol Assess Ser. 2025. PMID: 40727261 Free PMC article.
Cited by
-
Introduction to the Special Issue on Molecular Guided Surgery.J Biomed Opt. 2025 Jan;30(Suppl 1):S13701. doi: 10.1117/1.JBO.30.S1.S13701. Epub 2025 May 7. J Biomed Opt. 2025. PMID: 40343094 Free PMC article.
References
-
- Leibovitch I., et al. , “Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia I. Experience over 10 years,” J. Am. Acad. Dermatol. 53, 253–260 (2005).JAADDB - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

