Nonlinear Investigation of Fluorene-Benzothiadiazole Copolymers with Multiphoton Absorption and Highlights as Optical Limiters
- PMID: 40321589
- PMCID: PMC12044456
- DOI: 10.1021/acsomega.4c11627
Nonlinear Investigation of Fluorene-Benzothiadiazole Copolymers with Multiphoton Absorption and Highlights as Optical Limiters
Erratum in
-
Erratum: Corretion to "Nonlinear Investigation of Fluorene-Benzothiadiazole Copolymers with Multiphoton Absorption and Highlights as Optical Limiters".ACS Omega. 2025 Jul 30;10(31):35309. doi: 10.1021/acsomega.5c06629. eCollection 2025 Aug 12. ACS Omega. 2025. PMID: 40821537 Free PMC article.
Abstract
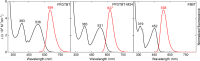
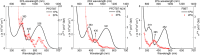
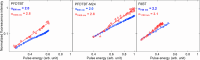
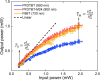
In recent years, conjugated polymers have garnered significant interest due to their versatile optical and electronic properties, including low band gaps and strong absorption in the visible and near-infrared regions. These features, combined with high molar absorptivity and notable photoluminescence and electroluminescence quantum yields, make these materials highly suitable for applications in optoelectronics, nonlinear optics, and other advanced photonic technologies. This study investigates the linear and nonlinear optical properties of three fluorene-benzothiadiazole-based copolymers-PFDTBT, PFDTBT-M24, and F8BT-differentiated by their electron-accepting units and polymer chain lengths. Through comprehensive spectroscopic analysis, including one-photon absorption, fluorescence emission, and multiphoton absorption studies, as well as quantum chemical calculations, the research provides insights into how molecular design can be optimized for nonlinear optical performance. The results reveal significant two-photon absorption cross sections and demonstrate the potential of these materials for multiphoton-excited fluorescence and optical limiting applications.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Zhu Y.; Champion R. D.; Jenekhe S. A. Conjugated donor-acceptor copolymer semiconductors with large intramolecular charge transfer: Synthesis, optical properties, electrochemistry, and field effect carrier mobility of thienopyrazine-based copolymers. Macromolecules 2006, 39, 8712–8719. 10.1021/ma061861g. - DOI
-
- Pıravadılı S.; et al. Fluorene-based donor-acceptor-type multifunctional polymer with bicarbazole pendant moiety for optoelectronic applications. J. Polym. Sci. 2021, 59, 1829–1840. 10.1002/pol.20210221. - DOI
-
- Patil A. O.; Heeger A. J.; Wudl F. Optical Properties of Conducting Polymers. Chem. Rev. 1988, 88, 183–200. 10.1021/cr00083a009. - DOI
-
- Decher G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. 10.1126/science.277.5330.1232. - DOI
LinkOut - more resources
Full Text Sources
