Gut Microbiota and Immunoglobulin A Nephropathy: Exploration of Dietary Intervention and Treatment Strategies
- PMID: 40321610
- PMCID: PMC12045934
- DOI: 10.1002/fsn3.70218
Gut Microbiota and Immunoglobulin A Nephropathy: Exploration of Dietary Intervention and Treatment Strategies
Abstract
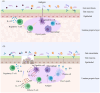
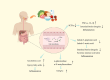
Immunoglobulin A nephropathy (IgAN) is a primary glomerular disease characterized by the deposition of IgA. The pathogenesis of it is related to the dysbiosis of gut microbiota. Dysbiosis of gut microbiota influences mucosal immune response and systemic immune system, leading to glycosylation-deficient IgA1 (Gd-IgA1) increasing, which promotes the development of IgAN. Diet plays an important role in regulating gut microbiota and treating IgAN. In this review, we summarize the interplay between gut microbiota and IgAN, and their underlying mechanisms. We also describe the effects of dietary intake on IgAN, as well as the composition of gut microbiota. The progress on IgAN treatment mainly focuses on inhibiting or regulating the immune system. Moreover, therapeutic strategies related to gut microbiota such as dietary intervention, supplement of probiotics and prebiotics, as well as fecal microbiota transplantation (FMT) have shown the possibility of improving IgAN prognosis. Thus, exploration of the gut-kidney axis, the long-term effects of diet and microbiome is necessary to develop more effective treatment strategies.
Keywords: IgAN; diet intervention; gut microbiota; treatment of IgAN.
© 2025 The Author(s). Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Is There a Role for Gut Microbiome Dysbiosis in IgA Nephropathy?Microorganisms. 2022 Mar 22;10(4):683. doi: 10.3390/microorganisms10040683. Microorganisms. 2022. PMID: 35456735 Free PMC article. Review.
-
IgA nephropathy: gut microbiome regulates the production of hypoglycosilated IgA1 via the TLR4 signaling pathway.Nephrol Dial Transplant. 2024 Sep 27;39(10):1624-1641. doi: 10.1093/ndt/gfae052. Nephrol Dial Transplant. 2024. PMID: 38402460 Free PMC article.
-
Characteristics, pathogenic and therapeutic role of gut microbiota in immunoglobulin A nephropathy.Front Immunol. 2025 Feb 6;16:1438683. doi: 10.3389/fimmu.2025.1438683. eCollection 2025. Front Immunol. 2025. PMID: 39981255 Free PMC article. Review.
-
Aberrant Gut Microbiome Contributes to Barrier Dysfunction, Inflammation, and Local Immune Responses in IgA Nephropathy.Kidney Blood Press Res. 2023;48(1):261-276. doi: 10.1159/000528973. Epub 2023 Mar 6. Kidney Blood Press Res. 2023. PMID: 36878203 Free PMC article.
-
Metagenomics-based systematic analysis reveals that gut microbiota Gd-IgA1-associated enzymes may play a key role in IgA nephropathy.Front Mol Biosci. 2022 Aug 24;9:970723. doi: 10.3389/fmolb.2022.970723. eCollection 2022. Front Mol Biosci. 2022. PMID: 36090029 Free PMC article.
References
-
- Aluko, E. O. , Nna V., and Adekunbi D.. 2015. “The Possible Mechanisms Through Which Dietary Protein Increases Renal Blood Flow and Glomerular Filtration Rate.” British Journal of Medicine & Medical Research 7, no. 6: 458–469. 10.9734/BJMMR/2015/16214. - DOI
-
- Antushevich, H. 2020. “Fecal Microbiota Transplantation in Disease Therapy.” Clinica Chimica Acta 503: 90–98. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

