Differences in the role of Gper1 in colorectal cancer progression depending on sex
- PMID: 40321663
- PMCID: PMC12046377
- DOI: 10.3892/ol.2025.15051
Differences in the role of Gper1 in colorectal cancer progression depending on sex
Abstract
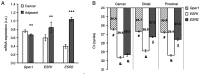
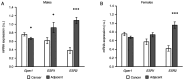
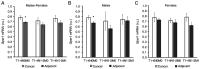
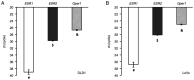
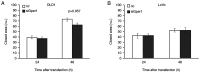
To evaluate the role of 17β-oestradiol (E2) in the sex-dependent progression of colorectal cancer (CRC), the present study focused on E2 signalling mediated via the nuclear receptors [oestrogen receptor (ESR)1 and ESR2] and the membrane G protein-coupled oestrogen receptor 1 (Gper1) in males and females diagnosed with CRC. This study also investigated Gper1 signalling in the CRC cell lines DLD1 and LoVo, which differ in the p53 pathway. In cancer tissue, Gper1 becomes by far the most abundant E2 receptor due to an increase in Gper1 and a decrease in ESR2 expression. These changes are more prominent in males than in females. More pronounced differences in Gper1 expression between cancer and adjacent tissues were observed in males in lower stages compared with those in higher stages of disease and females. High expression of Gper1 was associated with worse survival in males without nodal involvement but not in females. The expression of E2 receptors in the CRC cell lines DLD1 and LoVo resembles that of human cancer tissue. Silencing of Gper1 (siGper1) caused an increase in the rate of metabolism in LoVo cells with wild-type tp53. In DLD1 cells with the mutated form of tp53, siGper1 did not exert this effect. High levels of Gper1 were associated with worse survival and could contribute to sex-dependent changes in the CRC prognosis. Tumour suppressor effects of Gper1 were, at least to some extent, dependent on signalling downstream of p53, which was more frequently deficient in males than in females. Overall, this suggests that up-regulation of Gper1 (or administration of a Gper1 agonist) would be more beneficial for patients with wild-type tp53.
Keywords: DLD1; G protein-coupled receptor 30; LoVo; oestradiol; oestrogen receptor 1; oestrogen receptor 2; sex; survival.
Copyright: © 2025 Herichová et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
The GPER1/SPOP axis mediates ubiquitination-dependent degradation of ERα to inhibit the growth of breast cancer induced by oestrogen.Cancer Lett. 2021 Feb 1;498:54-69. doi: 10.1016/j.canlet.2020.10.019. Epub 2020 Oct 15. Cancer Lett. 2021. PMID: 33069770
-
Effect of miR-34a on the expression of clock and clock-controlled genes in DLD1 and Lovo human cancer cells with different backgrounds with respect to p53 functionality and 17β-estradiol-mediated regulation.PLoS One. 2023 Oct 13;18(10):e0292880. doi: 10.1371/journal.pone.0292880. eCollection 2023. PLoS One. 2023. PMID: 37831728 Free PMC article.
-
Environmental polycyclic aromatic hydrocarbons mixture, in human blood levels, decreased oestradiol secretion by granulosa cells via ESR1 and GPER1 but not ESR2 receptor.Hum Exp Toxicol. 2020 Mar;39(3):276-289. doi: 10.1177/0960327119886027. Epub 2019 Nov 7. Hum Exp Toxicol. 2020. PMID: 31698960
-
G protein-coupled oestrogen receptor 1 (GPER1)/GPR30: a new player in cardiovascular and metabolic oestrogenic signalling.Br J Pharmacol. 2011 Jul;163(6):1131-9. doi: 10.1111/j.1476-5381.2011.01235.x. Br J Pharmacol. 2011. PMID: 21250980 Free PMC article. Review.
-
Oestrogen and colorectal cancer: mechanisms and controversies.Int J Colorectal Dis. 2013 Jun;28(6):737-49. doi: 10.1007/s00384-012-1628-y. Epub 2013 Jan 15. Int J Colorectal Dis. 2013. PMID: 23319136 Review.
References
-
- Cardoso R, Guo F, Heisser T, Hackl M, Ihle P, De Schutter H, Van Damme N, Valerianova Z, Atanasov T, Májek O, et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: An international population-based study. Lancet Oncol. 2021;22:1002–1013. doi: 10.1016/S1470-2045(21)00199-6. - DOI - PubMed
-
- Murphy N, Ward HA, Jenab M, Rothwell JA, Boutron-Ruault MC, Carbonnel F, Kvaskoff M, Kaaks R, Kühn T, Boeing H, et al. Heterogeneity of colorectal cancer risk factors by anatomical subsite in 10 european countries: Amultinational cohort study. Clin Gastroenterol Hepatol. 2019;17:1323–1331.e6. doi: 10.1016/j.cgh.2018.07.030. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
