Adalimumab Biosimilars Demonstrate Long-Term Durability and Cost-Effectiveness in Paediatric Inflammatory Bowel Disease: A Real-World Two-Centre European Cohort Study
- PMID: 40321667
- PMCID: PMC12049108
- DOI: 10.2147/BTT.S511248
Adalimumab Biosimilars Demonstrate Long-Term Durability and Cost-Effectiveness in Paediatric Inflammatory Bowel Disease: A Real-World Two-Centre European Cohort Study
Abstract
Purpose: Adalimumab biosimilars are increasingly used in paediatric Inflammatory Bowel Disease (PIBD), but data remain limited. This study assessed their durability, efficacy, safety and cost implications in PIBD.
Patients and methods: Consecutive PIBD patients who started adalimumab biosimilars between October 2018 and December 2023 at two centres in Scotland and Italy, with at least 6 months follow-up, were included. Demographic, disease, treatment, and adverse event data were collected. Disease activity was assessed at baseline, 6, 12, 24, 36 months, and at last follow-up. Durability was evaluated using Kaplan-Meier analysis.
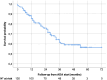
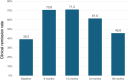
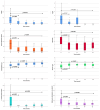
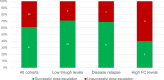
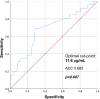
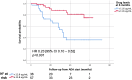
Results: In total 130 patients (81 males; median age 12.3 years) were included (115 Crohn's Disease, 7 Ulcerative Colitis, 8 IBD unclassified). The biosimilars were ABP 501 (85%), GP2017 (14%), SB5 (1%); 41 (32%) patients switched from originator. After a median follow-up of 26 months, 87/130 (67%) patients remained on biosimilars, while 43 discontinued at a median of 14 months. Durability probabilities were 93%, 86%, 75%, 62%, and 57% at 6, 12, 24, 36, and 54 months, respectively. Patients previously exposed to ADA originator had a lower risk of biosimilar failure (hazard ratio, adjusted for age at diagnosis: 0.51 [95% confidence interval: 0.26-0.99], p=0.047). Trough levels ≥11.6 μg/mL at 6 months were associated with greater durability (AUC=0.68, p=0.007). Adverse events occurred in 46/130 patients, mainly psoriasis and injection site reactions (13% each), with one lymphoma. Estimated cost savings were 5,030€ per patient/year.
Conclusion: This real-life study demonstrated high durability and remission rates for adalimumab biosimilars in PIBD, confirming their clinical, cost-effectiveness and safety profile in children.
Keywords: adalimumab; biosimilar; paediatric inflammatory bowel disease.
© 2025 Ancona et al.
Conflict of interest statement
SA fellowship is supported by University of Genoa. RKR has received speaker’s fees, travel support, or has performed consultancy work with: Nestle Health Sciences, AbbVie, Pharmacosmos, Lilly, Celltrion Healthcare, Ferring, Janssen & Pfizer. The remaining authors report no conflicts of interest in this work.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials

