This is a preprint.
The transcription factor HHEX maintains glucocorticoid levels and protects adrenals from androgen-induced lipid depletion
- PMID: 40321776
- PMCID: PMC12047992
- DOI: 10.21203/rs.3.rs-6248794/v1
The transcription factor HHEX maintains glucocorticoid levels and protects adrenals from androgen-induced lipid depletion
Abstract
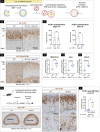
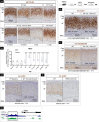
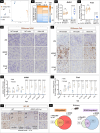
Glucocorticoid-producing cells of the adrenal cortex (i.e. zona fasciculata, zF) constitute the critical effectors of the hypothalamic-pituitary-adrenal axis, mediating the mammalian stress response. With glucocorticoids being essential for life, it is not surprising that zF dysfunction perturbs multiple organs that participate in optimizing cardiometabolic fitness. The zF forms a dynamic and heterogenous cell population endowed with the capacity to remodel through the engagement of both proliferative and differentiation programs that enable the adrenal to adapt and respond to diverse stressors. However, the mechanisms that sustain such differential responsiveness remain poorly understood. In this study, we resolved the transcriptome of the steroidogenic lineage by scRNA-seq using Sf1-Cre; Rosa mT/mG reporter mice. We identified HHEX, a homeodomain protein, as the most enriched transcription factor in glucocorticoid-producing cells. We developed new genetic mouse models to demonstrate that HHEX deletion causes glucocorticoid insufficiency in male animals. Molecularly, we demonstrated that HHEX is an androgen receptor (AR) target gene, shaping the sexual dimorphism of the adrenal gland by repressing the female transcriptional program at puberty, while also maintaining zF cholesterol ester content by protecting lipid droplets from androgen-induced-lipophagy. Moreover, our study revealed that, in both sexes, HHEX is crucial for maintaining the identity of the innermost adrenocortical cell subpopulation. Specifically, loss of HHEX impairs the expression of Abcb1b (P-glycoprotein/MDR1), an efflux pump regulating steroid export and cellular levels of xenobiotics. Together, these data demonstrate that HHEX serves as a multi-functional regulator of post-natal adrenal maturation that is potentiated by androgens.
Keywords: ACTH; HPA axis; PRH; Steroidogenic lineage; autophagy; cholesterol; corticosteroid; endocrine; lipid droplet; lipophagy; sexual dimorphism; stress response; testosterone; zona fasciculata.
Conflict of interest statement
Competing interest G.D.H. Founder and Board of Directors – Sling Therapeutics, Advisor – Orphagen Pharmaceuticals
Figures



Similar articles
-
Increased adrenal steroidogenesis and suppressed corticosteroid responsiveness in critical COVID-19.Metabolism. 2024 Nov;160:155980. doi: 10.1016/j.metabol.2024.155980. Epub 2024 Jul 23. Metabolism. 2024. PMID: 39053691
-
Development of sexual dimorphism of skeletal muscles through the adrenal cortex, caused by androgen-induced global gene suppression.Cell Rep. 2024 Feb 27;43(2):113715. doi: 10.1016/j.celrep.2024.113715. Epub 2024 Feb 1. Cell Rep. 2024. PMID: 38306273
-
Adrenocorticotropin-dependent changes in SF-1/DAX-1 ratio influence steroidogenic genes expression in a novel model of glucocorticoid-producing adrenocortical cell lines derived from targeted tumorigenesis.Endocrinology. 2006 Apr;147(4):1805-18. doi: 10.1210/en.2005-1279. Epub 2006 Jan 26. Endocrinology. 2006. PMID: 16439455
-
Secretin, glucagon, gastric inhibitory polypeptide, parathyroid hormone, and related peptides in the regulation of the hypothalamus- pituitary-adrenal axis.Peptides. 2000 Feb;21(2):309-24. doi: 10.1016/s0196-9781(99)00193-x. Peptides. 2000. PMID: 10764961 Review.
-
Glucocorticoid metabolism in the developing lung: adrenal-like synthesis pathway.J Steroid Biochem Mol Biol. 2013 Nov;138:72-80. doi: 10.1016/j.jsbmb.2013.03.004. Epub 2013 Mar 26. J Steroid Biochem Mol Biol. 2013. PMID: 23537622 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

