This is a preprint.
Successful insertion and expression of a tetracycline transactivator in Anopheles stephensi associated with increased egg production and decreased hatching rate
- PMID: 40321780
- PMCID: PMC12047982
- DOI: 10.21203/rs.3.rs-6270709/v1
Successful insertion and expression of a tetracycline transactivator in Anopheles stephensi associated with increased egg production and decreased hatching rate
Update in
-
Successful insertion and expression of a tetracycline transactivator in Anopheles stephensi associated with increased egg production and decreased hatching rate.Parasit Vectors. 2025 Oct 28;18(1):433. doi: 10.1186/s13071-025-07003-7. Parasit Vectors. 2025. PMID: 41152980 Free PMC article.
Abstract
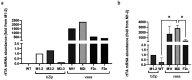
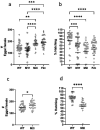
Sanaria® has pioneered production of aseptic, purified, vialed cryopreserved Plasmodium falciparum (Pf) sporozoites (SPZ) as vaccines and for controlled human malaria infections. More than 3,500 individuals have received more than 9,700 injections of PfSPZ, worldwide. The PfSPZ are manufactured in aseptically reared female Anopheles stephensi mosquitoes. Since PfSPZ vaccines are intended primarily for some of the most disadvantaged people in the world, keeping costs low is imperative. One approach to reducing cost of goods is to eliminate male mosquitoes from the production process, thereby doubling the numbers of PfSPZ-producing mosquitoes per unit space. We intend to do this by creating A. stephensi with a male-lethal allele controlled by the tetracycline conditional gene expression system. Herein, we report the first step in this process, the creation of a driver line that expresses the reverse tetracycline transactivator (rtTA). After sub-optimal results using the bZip early embryonic promoter, we produced 3 mosquito driver lines that expressed rtTA from 3 different genomic loci under the early embryonic vasa promoter. Expressing the rtTA under the vasa promoter significantly increased rtTA mRNA levels compared to its level under bZip. We were unable to achieve homozygosity in two of these lines even after 26 generations. In the third line, the insertion was in an intron of a putative juvenile hormone diol kinase gene. Homozygosity was reached in this line after passage through 7 generations, indicating that the inserted vasa-rtTA nucleotides did not interfere with the function of an essential genomic locus. rtTA mRNA expression levels were higher than in the other two lines, supporting the hypothesis that failure to achieve homozygosity was not due to rtTA expression but to insertion position. The homozygous line produced ~ 18% more eggs per female and a hatching rate of larvae from eggs was 39% lower than of wild-type A. stephensi. The next step will be to cross the driver line with an effector line containing a male-linked lethal gene regulated by the tetracycline responsive element (TRE).
Keywords: Anopheles; Tetracycline; driver line; rtTA.
Conflict of interest statement
Competing interests E.I., A.E.G., T.V.P, G.J., P.F.B., and S.L.H. are salaried employees of Sanaria Inc., the developer and owner of PfSPZ vaccines, and A.E.G. and P.F.B. were salaried employees of Sanaria Inc. when the work was performed. S.L.H. has a financial interest in Sanaria Inc. No other authors have competing interests.
Figures


References
-
- Vector-borne diseases [Internet]. World Health Organization; 2024. [cited 09/26/2024]. Available from: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases#:....
-
- Hoffman SL, Billingsley PF, James E, Richman A, Loyevsky M, Li T, et al. Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum Vaccin. 2010;6(1):97–106. - PubMed
-
- Sissoko MS, Healy SA, Katile A, Omaswa F, Zaidi I, Gabriel EE, et al. Safety and efficacy of PfSPZ Vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: a randomised, double-blind phase 1 trial. Lancet Infect Dis. 2017;17(5):498–509. - PMC - PubMed
-
- Olotu A, Urbano V, Hamad A, Eka M, Chemba M, Nyakarungu E, et al. Advancing Global Health through Development and Clinical Trials Partnerships: A Randomized, Placebo-Controlled, Double-Blind Assessment of Safety, Tolerability, and Immunogenicity of Plasmodium falciparum Sporozoites Vaccine for Malaria in Healthy Equatoguinean Men. Am J Trop Med Hyg. 2018;98(1):308–18. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

