Protective antibody response in Korean raccoon dogs (Nyctereutes procynoide koreensis) administered a new rabies bait vaccine containing the ERAGS-GFP strain
- PMID: 40321792
- PMCID: PMC12046086
- DOI: 10.7774/cevr.2025.14.e13
Protective antibody response in Korean raccoon dogs (Nyctereutes procynoide koreensis) administered a new rabies bait vaccine containing the ERAGS-GFP strain
Abstract
Purpose: Rabies is a deadly zoonotic disease affecting many mammals, including humans. Oral rabies bait vaccines induce an immune response without direct inoculation, and are crucial for controlling rabies in wildlife. This study evaluated the safety and immunogenicity of a new rabies bait vaccine containing a recombinant rabies virus expressing green fluorescent protein (ERAGS-GFP) in wild raccoon dogs.
Materials and methods: To confirm the safety of the ERAGS-GFP vaccine, reversion to virulence was evaluated in 1-day-old suckling mice. The uptake, minimum effective dose, and immunogenicity of the bait vaccine were assessed in raccoon dogs, as was the persistence of post-vaccine immunity. Serum rabies virus neutralizing antibody (VNA) titers were measured using fluorescent antibody virus neutralization.
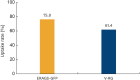
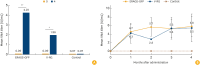
Results: No adverse effects were noted in mice, guinea pigs, dogs, or raccoon dogs administered the ERAGS-GFP vaccine orally during the test period. The glycoprotein gene of the ERAGS-GFP strain remained unchanged after five reverse passages in 1-day-old mice. Uptake of the bait vaccine was 75.8% in raccoon dogs. The minimum effective dose was at least 105.0 TCID50/mL. Forty-three raccoon dogs administered the ERAGS-GFP bait vaccine developed an average VNA titer of 4.23 IU/mL 28 days post-administration. Protective antibody levels were maintained for 4 months.
Conclusion: The ERAGS-GFP bait vaccine showed high uptake and strong immunogenicity in raccoon dogs, and protective antibody levels were maintained for at least 4 months. These results indicate the vaccine's potential for effective rabies control in wildlife, which can reduce the risk of transmission to humans and domestic animals.
Keywords: Bait; ERAGS-GFP; Immunologyy; Rabies; Raccoon dogs.
© Korean Vaccine Society, Korean Society for Zoonoses.
Conflict of interest statement
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Figures



Similar articles
-
Safety and immunogenicity of recombinant rabies virus (ERAGS) in mice and raccoon dogs.Clin Exp Vaccine Res. 2016 Jul;5(2):159-68. doi: 10.7774/cevr.2016.5.2.159. Epub 2016 Jul 29. Clin Exp Vaccine Res. 2016. PMID: 27489806 Free PMC article.
-
Immunogenicity of an inactivated rabies vaccine for animals derived from the recombinant ERAGS strain.Clin Exp Vaccine Res. 2021 May;10(2):141-147. doi: 10.7774/cevr.2021.10.2.141. Epub 2021 May 31. Clin Exp Vaccine Res. 2021. PMID: 34222126 Free PMC article.
-
A recombinant rabies virus (ERAGS) for use in a bait vaccine for swine.Clin Exp Vaccine Res. 2016 Jul;5(2):169-74. doi: 10.7774/cevr.2016.5.2.169. Epub 2016 Jul 29. Clin Exp Vaccine Res. 2016. PMID: 27489807 Free PMC article.
-
Efficacy and bait acceptance of vaccinia vectored rabies glycoprotein vaccine in captive foxes (Vulpes vulpes), raccoon dogs (Nyctereutes procyonoides) and dogs (Canis familiaris).Vaccine. 2008 Aug 26;26(36):4627-38. doi: 10.1016/j.vaccine.2008.06.089. Epub 2008 Jul 11. Vaccine. 2008. PMID: 18620017 Review.
-
Oral vaccination of wildlife using a vaccinia-rabies-glycoprotein recombinant virus vaccine (RABORAL V-RG®): a global review.Vet Res. 2017 Sep 22;48(1):57. doi: 10.1186/s13567-017-0459-9. Vet Res. 2017. PMID: 28938920 Free PMC article. Review.
References
-
- Cliquet F, Gurbuxani JP, Pradhan HK, et al. The safety and efficacy of the oral rabies vaccine SAG2 in Indian stray dogs. Vaccine. 2007;25:3409–3418. - PubMed
-
- Schumacher CL, Coulon P, Lafay F, et al. SAG-2 oral rabies vaccine. Onderstepoort J Vet Res. 1993;60:459–462. - PubMed
-
- Orciari LA, Niezgoda M, Hanlon CA, et al. Rapid clearance of SAG-2 rabies virus from dogs after oral vaccination. Vaccine. 2001;19:4511–4518. - PubMed
LinkOut - more resources
Full Text Sources

