Nano-Encapsulated Taro Lectin Can Cross an in vitro Blood-Brain Barrier, Induce Apoptosis and Autophagy and Inhibit the Migration of Human U-87 MG Glioblastoma Cells
- PMID: 40321803
- PMCID: PMC12049682
- DOI: 10.2147/IJN.S511506
Nano-Encapsulated Taro Lectin Can Cross an in vitro Blood-Brain Barrier, Induce Apoptosis and Autophagy and Inhibit the Migration of Human U-87 MG Glioblastoma Cells
Abstract
Background: Tarin, purified from taro (Colocasia esculenta), promotes anticancer effect against glioblastoma cells, a heterogeneous and aggressive primary central nervous system tumor and one of the most challenging tumors for oncotherapy. If able to overcome the blood-brain barrier (BBB), tarin may comprise a natural defense against glioblastomas in a context of the development of novel drugs to control these malignant cell proliferations.
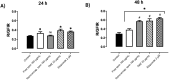
Methods: The anticancer effects of nano-encapsulated tarin were tested against U-87 MG cells and the molecular mechanisms involved in cell proliferation control were assessed by flow cytometry and transmission electron microscopy (TEM) analyses. The scratch assay was performed to investigate cell migration capacity, while nano-encapsulated tarin transport across the BBB was tested on the hCMEC/D3 endothelial cell line.
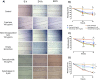
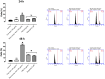
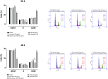
Results: Nano-encapsulated tarin induced autophagy in U-87 MG cells, characterized by the presence of autophagosomes as revealed by TEM and corroborating the flow cytometry analysis employing acridine orange. Additional ultrastructural changes, such as mitochondrial swelling, were also observed. The presence of apoptotic cells and caspase 3/7 activation indicate that nano-encapsulated tarin may also induce cell death through apoptosis. Glioblastoma cell proliferation was arrested in the G2/M cell cycle phase, and cell migration was delayed. Reduced cell proliferation and glioblastoma cell migration inhibition were significant, as tarin was efficiently transported across the BBB during in vitro assays.
Conclusion: Nano-encapsulated tarin may be effectively employed to inhibit glioblastoma cell proliferation and migration, as this novel formulation can overcome the BBB and induces carcinoma cell apoptosis and autophagy. Furthermore, nano-encapsulated tarin may comprise a novel chemotherapeutic agent against different tumoral lines, as it is able to control glioblastoma tumor proliferation by the same molecular mechanisms previously reported for breast adenocarcinomas. Additional studies should be carried out to clarify if nano-encapsulated tarin has a general effect on distinct carcinoma lines.
Keywords: Colocasia esculenta; GNA-related lectin; antitumoral lectin; caspase 3-7 activation; cell cycle arrest; transendothelial permeability.
© 2025 Cardoso et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures




Similar articles
-
Tarin-Loaded Nanoliposomes Activate Apoptosis and Autophagy and Inhibit the Migration of Human Mammary Adenocarcinoma Cells.Int J Nanomedicine. 2023 Nov 7;18:6393-6408. doi: 10.2147/IJN.S434626. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37954458 Free PMC article.
-
Liposomal Taro Lectin Nanocapsules Control Human Glioblastoma and Mammary Adenocarcinoma Cell Proliferation.Molecules. 2019 Jan 29;24(3):471. doi: 10.3390/molecules24030471. Molecules. 2019. PMID: 30699910 Free PMC article.
-
Cyclovirobuxine D inhibits cell proliferation and migration and induces apoptosis in human glioblastoma multiforme and low‑grade glioma.Oncol Rep. 2020 Mar;43(3):807-816. doi: 10.3892/or.2020.7459. Epub 2020 Jan 13. Oncol Rep. 2020. PMID: 32020219 Free PMC article.
-
Nano delivery of natural substances as prospective autophagy modulators in glioblastoma.Nanomedicine. 2020 Oct;29:102270. doi: 10.1016/j.nano.2020.102270. Epub 2020 Jul 21. Nanomedicine. 2020. PMID: 32702467 Review.
-
Overcoming the blood-brain tumor barrier for effective glioblastoma treatment.Drug Resist Updat. 2015 Mar;19:1-12. doi: 10.1016/j.drup.2015.02.002. Epub 2015 Mar 6. Drug Resist Updat. 2015. PMID: 25791797 Review.
References
-
- Uddin MS, Al Mamun A, Alghamdi BS, et al. Epigenetics of glioblastoma multiforme: from molecular mechanisms to therapeutic approaches. Paper presented at: Seminars in cancer biology. 2022. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

