Novel Antigen-Presenting Cell-Targeted Nanoparticles Enhance Split Vaccine Immunity Through Microneedles Inoculation
- PMID: 40321807
- PMCID: PMC12050023
- DOI: 10.2147/IJN.S502724
Novel Antigen-Presenting Cell-Targeted Nanoparticles Enhance Split Vaccine Immunity Through Microneedles Inoculation
Abstract
Aim/background: Despite their superior safety and widespread use, split vaccines typically suffer from reduced immunogenicity due to the lack of an intact viral structure. Targeting the mannose receptors on antigen-presenting cells (APCs) with nanoparticles (NPs) and delivering them via microneedles (MNs) offers a promising solution. We designed and synthesized NPs that could form complexes with split H1N1 antigens, and evaluated the immunogenicity after loading them into dissolvable microneedle arrays (dMAs).
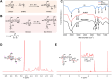
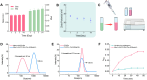
Methods: Man-N-HACC was synthesized by conjugating mannose moieties to N-2-hydroxypropyl trimethyl ammonium chloride chitosan (N-HACC), followed by cross-linking with tripolyphosphate to form Man-N-HACC NPs. The NPs were characterized in terms of morphology, size, zeta potential, spatial orientation, macrophage internalization, and stability. The microstructure, mechanical strength, skin penetration capability, and release behavior of dMAs loaded with Man-N-HACC NPs/H1N1 complexes were investigated. Finally, the efficacy of dMAs was assessed in a rat model using ELISA and hemagglutination inhibition (HAI) assay.
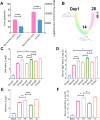
Results: Characterization via Fourier transform infrared spectroscopy and nuclear magnetic resonance confirmed the synthesis of Man-N-HACC. The cross-linked generated Man-N-HACC NPs displayed uniform morphology and good stability over 28 days, along with confirmed spatial orientation of mannose ligands and macrophage internalization. The dMAs loaded with Man-N-HACC NPs/H1N1 exhibited mechanical robustness, capable of fully penetrating the skin and releasing nanovaccines. The increase in HAI titers and total IgG antibody levels in rat serum indicates the effectiveness of humoral immunity, and this effect only occurs after NPs formed post-crosslinking, rather than directly using raw nanomaterials, highlighting the critical role of the nanoparticle structure.
Conclusion: This study confirms that the delivery of Man-N-HACC NPs via dMAs provides a novel and promising approach for the administration of split influenza vaccines. Moreover, it underscores the great potential of nano-adjuvants in enhancing the efficacy of split vaccines.
Keywords: dissolvable microneedle arrays; mannose receptor; nanoparticles; split vaccine; target.
© 2025 Xiu et al.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Liu T, Luo G, Xing M. Biomedical applications of polymeric microneedles for transdermal therapeutic delivery and diagnosis: current status and future perspectives. Adv Ther. 2020;3(9). doi: 10.1002/adtp.201900140 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

