FLIMB: fluorescence lifetime microendoscopy for metabolic and functional imaging of femoral marrow at subcellular resolution
- PMID: 40321997
- PMCID: PMC12047734
- DOI: 10.1364/BOE.549311
FLIMB: fluorescence lifetime microendoscopy for metabolic and functional imaging of femoral marrow at subcellular resolution
Abstract
Intravital imaging of bone marrow provides a unique opportunity to study cellular dynamics and their interaction with the tissue microenvironment, which governs cell functions and metabolic profiles. To optically access the deep marrow of long bones, we previously developed a microendoscopy system for longitudinal two-photon fluorescence imaging of the murine femur. However, this does not provide information on cell functions or metabolism, for which quantification fluorescence lifetime imaging (FLIM) has proven to be a versatile tool. We present and characterize FLIMB, an adapted GRIN-based microendoscopic system capable of performing reliable, co-registered TCSPC-based two-photon excited FLIM and fluorescence imaging in the femur of fluorescent reporter mice, at sub-cellular resolution. Using FLIMB, we demonstrate metabolic imaging via NAD(P)H-FLIM and intracellular Ca2+ signaling via FRET-FLIM in immune cell subsets, in the femoral marrow. This method retains the power to study molecular mechanisms underlying various cell functions in tissue context thus providing new insights into bone biology.
© 2025 Optica Publishing Group.
Conflict of interest statement
Heribert Stürmer is employed by GRINTech GmbH, Jena, Germany. Romano Matthys and Reto Nützi are with RISystem, Landquart, Switzerland. All other authors declare no conflict of interest.
Figures

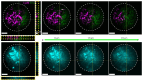
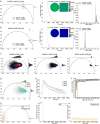
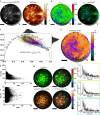


References
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous
