Air-pulse optical coherence elastography: how excitation angle affects mechanical wave propagation
- PMID: 40322015
- PMCID: PMC12047731
- DOI: 10.1364/BOE.557984
Air-pulse optical coherence elastography: how excitation angle affects mechanical wave propagation
Abstract
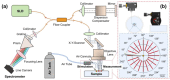
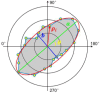
We evaluate the effect of excitation angles on the observation and characterization of surface wave propagations used to derive tissue's mechanical properties in optical coherence tomography (OCT)-based elastography (OCE). Air-pulse stimulation was performed at the center of the sample with excitation angles ranging from oblique (e.g., 70° or 45°) to perpendicular (0°). OCT scanning was conducted radially to record en face mechanical wave propagations in 360°, and the wave features (amplitude, attenuation, group and phase velocities) were calculated in the spatiotemporal or wavenumber-frequency domains. We conducted measurements on isotropic, homogeneous samples (1-1.6% agar phantoms), anisotropic samples (chicken breast), and samples with complex boundaries, coupling media, and stress conditions (ex vivo porcine cornea, intraocular pressure (IOP): 5-20 mmHg). Our findings indicate that mechanical wave velocities are less affected by excitation angles compared to displacement features, demonstrating the robustness of using mechanical waves for elasticity estimations. Agar and chicken breast sample measurements showed that all these metrics (particularly wave velocities) are relatively consistent when excitation angles are smaller than 45°. However, significant disparities were observed in the porcine cornea measurements across different excitation angles (even between 15° and 0°), particularly at high IOP levels (e.g., 20 mmHg). Our findings provide valuable insights for enhancing the accuracy of biomechanical assessments using air-pulse-based or other dynamic OCE approaches. This facilitates the refinement and clinical translation of the OCE technique and could ultimately improve diagnostic and therapeutic applications across various biomedical fields.
© 2025 Optica Publishing Group.
Conflict of interest statement
GL, JX and YH: Weiren Meditech Co., Ltd. (C); JQ. and LA: Weiren Meditech Co., Ltd. (E).
Figures







Similar articles
-
Chirp excitation for natural frequency optical coherence elastography.Biomed Opt Express. 2024 Sep 13;15(10):5856-5871. doi: 10.1364/BOE.536685. eCollection 2024 Oct 1. Biomed Opt Express. 2024. PMID: 39421777 Free PMC article.
-
Micro Air-Pulse Spatial Deformation Spreading Characterizes Degree of Anisotropy in Tissues.IEEE J Sel Top Quantum Electron. 2021 Jul-Aug;27(4):6800810. doi: 10.1109/jstqe.2020.3038633. Epub 2020 Nov 17. IEEE J Sel Top Quantum Electron. 2021. PMID: 33994766 Free PMC article.
-
Noncontact Elastic Wave Imaging Optical Coherence Elastography for Evaluating Changes in Corneal Elasticity Due to Crosslinking.IEEE J Sel Top Quantum Electron. 2016 May-Jun;22(3):6801911. doi: 10.1109/JSTQE.2015.2510293. Epub 2015 Dec 17. IEEE J Sel Top Quantum Electron. 2016. PMID: 27547022 Free PMC article.
-
Optical coherence elastography and its applications for the biomechanical characterization of tissues.J Biophotonics. 2023 Dec;16(12):e202300292. doi: 10.1002/jbio.202300292. Epub 2023 Oct 9. J Biophotonics. 2023. PMID: 37774137 Review.
-
Optical coherence elastography in ophthalmology.J Biomed Opt. 2017 Dec;22(12):1-28. doi: 10.1117/1.JBO.22.12.121720. J Biomed Opt. 2017. PMID: 29275544 Free PMC article. Review.
References
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
