Perspective on an Innovative Curative Strategy for Peritoneal Metastasis Involving Peritonectomy, Hyperthermic Intraperitoneal Chemotherapy, and Adjuvant Chemotherapy Identified as Effective in the Histoculture Drug Response Assay (HDRA)
- PMID: 40322213
- PMCID: PMC12046657
- DOI: 10.21873/cdp.10440
Perspective on an Innovative Curative Strategy for Peritoneal Metastasis Involving Peritonectomy, Hyperthermic Intraperitoneal Chemotherapy, and Adjuvant Chemotherapy Identified as Effective in the Histoculture Drug Response Assay (HDRA)
Abstract
Background/aim: Peritoneal carcinomatosis is the end stage for patients with gastrointestinal cancer, with survival ranging between 2 and 9 months. Pancreatic acinar cell carcinoma (PACC) is rare and can result in peritoneal metastases. The efficacy of chemotherapy for patients with PACC is unknown, and a systemic treatment strategy has not been established. The aim of the present perspective is to discuss a potential curative strategy combining surgery, heated intraperitoneal chemotherapy (HIPEC), and the histoculture drug response assay (HDRA) to identify effective adjuvant chemotherapy for PACC with peritoneal metastases, based on a published case report.
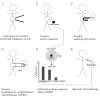
Case report: A 31-year-old man with a 20 cm epigastric mass, diagnosed as PACC, had curative-intent resection of a tumor on the distal stomach and pancreas tail. The patient recurred after four courses of adjuvant oral S-1 treatment. Laparotomy demonstrated peritoneal metastases with a peritoneal cancer index of 18. Ascites or other cancer cells in the peritoneal wash were not found. Peritonectomy, combined with HIPEC with gemcitabine and docetaxel, was performed intraoperatively. Postoperative 3-dimensional histoculture of fragments of the resected tumor with drug response testing with the histoculture drug response assay (HDRA) showed gemcitabine had the highest tumor inhibitory rate (70%) among six drugs tested. Based on the HDRA results, the patient was treated with adjuvant systemic gemcitabine chemotherapy. The patient did not have a recurrence within 18 months after surgery.
Conclusion: The present innovative treatment of PACC with peritoneal metastases used laparotomy to determine the extent of peritoneal metastases, peritonectomy to attempt to completely remove the tumor, HIPEC for intraoperative hyperthermic-chemotherapy, and the HDRA to determine the most effective drug for adjuvant chemotherapy. These procedures can be individualized for each patient's cancer, and the HDRA is most critical for individualization.
Keywords: HDRA; HIPEC; Peritoneal metastasis; adjuvant chemotherapy; cure; gemcitabine; histoculture drug response assay; hyperthermic intraperitoneal chemotherapy; pancreatic acinar cell carcinoma; peritonectomy.
©2025 The Author(s). Published by the International Institute of Anticancer Research.
Conflict of interest statement
The Authors declare no competing interests regarding this work.
Figures
References
-
- Yonemura Y, Ishibashi H, Fujita T, Liu Y, Wakama S, Sako S, Kitai T, Katayama K, Kamada Y, Taniguchi K, Fujimoto D, Kajiwara J, Hoffman RM. Pancreatic acinar cell carcinoma with peritoneal recurrence treated with peritonectomy and intraoperative hyperthermic intraperitoneal chemotherapy-a case report. Jpn. J Cancer Chemother. 2023;50(13):1931–1933. - PubMed
LinkOut - more resources
Full Text Sources