A passive blood separation sensing platform for point-of-care devices
- PMID: 40322246
- PMCID: PMC12048346
- DOI: 10.1038/s44328-025-00038-x
A passive blood separation sensing platform for point-of-care devices
Abstract
The blood test is one of the most performed investigations in clinical practice, with samples typically analysed in a centralised laboratory. Many of these tests monitor routine conditions that would benefit from a point-of-care approach, reducing the burden on practitioners, patients and healthcare systems. Such a decentralised model requires the development of sophisticated, yet easy-to-use technology; however, platforms that combine high-performance with low-cost and simplicity remain scarce. Moreover, most research papers only address a subset of requirements and study specific aspects in isolation. A systems approach that considers the interplay between each element of the technology is clearly required to develop a coherent solution. Here, we present such a systems approach in the context of testing for C-reactive protein (CRP), a commonly requested test in clinical practise that indicates inflammation and is particularly relevant for monitoring patients with chronic diseases, e.g. those with rheumatoid arthritis or who are undergoing cancer therapy. The approach we take here features an entirely passive microfluidic cartridge for blood separation, integrated with a high-performance sensing platform which we have tested in a real-world context. The device is compatible with a handheld detection unit and is simple to use yet can accurately detect CRP levels at clinically relevant levels.
Keywords: Applied optics; Biomedical engineering; Diagnostic markers; Lab-on-a-chip; Nanobiotechnology; Sensors and probes.
© The Author(s) 2025.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures

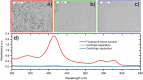
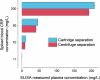

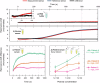
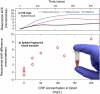
References
-
- Future of POC and Rapid Testing. (Ipsos, Sermo).
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
