Updates on Pulmonary Hypertension
- PMID: 40322494
- PMCID: PMC12046238
- DOI: 10.2174/0118743064344024250203101417
Updates on Pulmonary Hypertension
Abstract
Pulmonary Arterial Hypertension (PAH) is an uncommon condition with high mortality. It is an underrecognized condition both in developing and developed countries, especially in developing countries, due to a lack of advanced healthcare facilities and resources for timely diagnosis. More than half of the individuals diagnosed with PAH live less than five years after diagnosis. In recent years, tremendous advancements have been made in diagnostic and therapeutic strategies for PAH patients. Phosphodiesterase 5 (PDE5) inhibitors, endothelin receptor antagonists, and prostacyclin inhibitors in various forms (oral, inhaled, intravenous, or subcutaneous) have been the cornerstone of medical treatment. Atrial septostomy, heart and lung transplant, balloon pulmonary angioplasty, and pulmonary thromboendarterectomy are existing therapeutic options currently available. There has been a continuous effort to introduce newer therapies to improve life expectancy and modify disease. Newer therapies have shown promising results but require future data to guarantee long-term safety and efficacy. We aim to discuss a few of these critical updates in the constantly evolving field of PAH.
Keywords: Inhaled Treprostinil; Macitentan; Pulmonary hypertension; Remote pulmonary artery pressure monitoring; Sotatercept; Tadalafil.
© 2025 The Author(s). Published by Bentham Open.
Conflict of interest statement
Dr. Joseph Varon is Editor in Chief, Dr. Salim R. Surani is Co-editor in Chief, Dr. Munish Sharma is the Editorial Board Member of The Open Respiratory Medicine Journal.
Figures

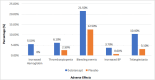
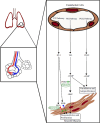

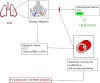

References
-
- Humbert M., Kovacs G., Hoeper M.M., Badagliacca R., Berger R.M.F., Brida M., Carlsen J., Coats A.J.S., Escribano-Subias P., Ferrari P., Ferreira D.S., Ghofrani H.A., Giannakoulas G., Kiely D.G., Mayer E., Meszaros G., Nagavci B., Olsson K.M., Pepke-Zaba J., Quint J.K., Rådegran G., Simonneau G., Sitbon O., Tonia T., Toshner M., Vachiery J.L., Vonk Noordegraaf A., Delcroix M., Rosenkranz S., Schwerzmann M., Dinh-Xuan A.T., Bush A., Abdelhamid M., Aboyans V., Arbustini E., Asteggiano R., Barberà J.A., Beghetti M., Čelutkienė J., Cikes M., Condliffe R., de Man F., Falk V., Fauchier L., Gaine S., Galié N., Gin-Sing W., Granton J., Grünig E., Hassoun P.M., Hellemons M., Jaarsma T., Kjellström B., Klok F.A., Konradi A., Koskinas K.C., Kotecha D., Lang I., Lewis B.S., Linhart A., Lip G.Y.H., Løchen M.L., Mathioudakis A.G., Mindham R., Moledina S., Naeije R., Nielsen J.C., Olschewski H., Opitz I., Petersen S.E., Prescott E., Rakisheva A., Reis A., Ristić A.D., Roche N., Rodrigues R., Selton-Suty C., Souza R., Swift A.J., Touyz R.M., Ulrich S., Wilkins M.R., Wort S.J., ESC/ERS Scientific Document Group 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022;43(38):3618–3731. doi: 10.1093/eurheartj/ehac237. - DOI - PubMed
-
- Wijeratne D.T., Lajkosz K., Brogly S.B., Lougheed M.D., Jiang L., Housin A., Barber D., Johnson A., Doliszny K.M., Archer S.L. Increasing incidence and prevalence of world health organization groups 1 to 4 pulmonary hypertension. Circ. Cardiovasc. Qual. Outcomes. 2018;11(2):e003973. doi: 10.1161/CIRCOUTCOMES.117.003973. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
