Thymosin β4 Regulates Tissue Inflammatory Response in Mouse Nonalcoholic Fatty Liver Disease by Promoting Macrophage M2-Type Polarization
- PMID: 40322536
- PMCID: PMC12049133
- DOI: 10.2147/JIR.S492814
Thymosin β4 Regulates Tissue Inflammatory Response in Mouse Nonalcoholic Fatty Liver Disease by Promoting Macrophage M2-Type Polarization
Abstract
Introduction: Nonalcoholic fatty liver disease (NAFLD) is characterized by hepatic steatosis, insulin resistance, and systemic pro-inflammatory response. Thymosin β4 (Tβ4) is a bioactive polypeptide that inhibits extracellular matrix (ECM) deposition and protects the liver. It can achieve immune homeostasis by regulating the polarization of liver macrophages and is a potential treatment for NAFLD.
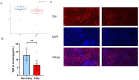
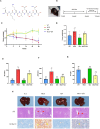
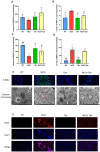
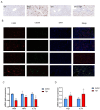
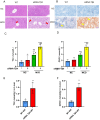
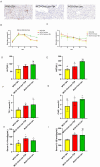
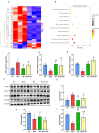
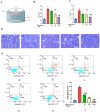
Methods: A dataset was used to evaluate the expression of Tβ4 in fatty and non-fatty adjacent tissues of primary hepatocellular carcinoma. NAFLD was induced in C57 mice with methionine and choline-deficient diet (MCD), siRNATβ4 was injected into the tail vein to reduce liver Tβ4, and the therapeutic effect of Tβ4 was observed by phagocytosis of macrophages with clodronate liposomes. Hematoxylin and Eosin staining (HE) staining was used to observe the inflammation of mice in each group, and oil red O staining was used to determine the lipid accumulation. Macrophage polarization was detected by immunofluorescence assay. In the extrachromosomal experiment of oil red O, human myeloid leukemia mononuclear (THP-1) cells was co-cultured with human hepatic (LO2) constructed with oleic acid to detect the changes of aspartate transaminase (AST) and alanine transaminase (ALT) in supernatant and the apoptosis of LO2 under the intervention of different concentrations of Tβ4.
Results: Tβ4 allowed the mice to recover from NAFLD and reduce liver inflammation more effectively. Liver steatosis was more severe in sirnat4 mice. Macrophages are involved in Tβ4 treatment of NAFLD. The expression level of M1 phenotype in macrophages treated with Tβ4 decreased, and the apoptosis of hepatocytes decreased. At the same time, Tβ4 down-regulates signal transduction and activator of transcription1 (STAT1) phosphorylation and increases suppressor of cytokine signaling1/3 (SOCS1/3) expression in hepatocytes.
Discussion: This study revealed the molecular mechanism of the effective effect of Tβ4 on the polarization of liver macrophages, suggesting that Tβ4 may be a potential therapeutic measure for NAFLD.
Keywords: NAFLD; inflammation; macrophage; thymosin beta 4.
© 2025 Zhu et al.
Conflict of interest statement
The authors declare no competing interests in this work.
Figures
Similar articles
-
Thymosin beta 4 alleviates non-alcoholic fatty liver by inhibiting ferroptosis via up-regulation of GPX4.Eur J Pharmacol. 2021 Oct 5;908:174351. doi: 10.1016/j.ejphar.2021.174351. Epub 2021 Jul 16. Eur J Pharmacol. 2021. PMID: 34280397
-
The expression of thymosin β4 in chronic hepatitis B combined nonalcoholic fatty liver disease.Medicine (Baltimore). 2016 Dec;95(52):e5763. doi: 10.1097/MD.0000000000005763. Medicine (Baltimore). 2016. PMID: 28033294 Free PMC article.
-
[Parathyroid hormone-related protein aggravates nonalcoholic fatty liver disease induced by methionine choline-deficient diet in mice].Nan Fang Yi Ke Da Xue Xue Bao. 2021 Jul 20;41(7):1037-1043. doi: 10.12122/j.issn.1673-4254.2021.07.10. Nan Fang Yi Ke Da Xue Xue Bao. 2021. PMID: 34308853 Free PMC article. Chinese.
-
Potential role of thymosin beta 4 in the treatment of nonalcoholic fatty liver disease.Chronic Dis Transl Med. 2017 Jul 8;3(3):165-168. doi: 10.1016/j.cdtm.2017.06.003. eCollection 2017 Sep. Chronic Dis Transl Med. 2017. PMID: 29063072 Free PMC article. Review.
-
Thymosin Beta 4 Is a Potential Regulator of Hepatic Stellate Cells.Vitam Horm. 2016;102:121-49. doi: 10.1016/bs.vh.2016.04.011. Epub 2016 May 31. Vitam Horm. 2016. PMID: 27450733 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

