Homogeneous antibody-drug conjugates with dual payloads: potential, methods and considerations
- PMID: 40322862
- PMCID: PMC12054377
- DOI: 10.1080/19420862.2025.2498162
Homogeneous antibody-drug conjugates with dual payloads: potential, methods and considerations
Abstract
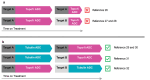
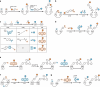
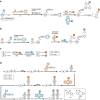
The development of site-specific dual-payload antibody-drug conjugates (ADCs) represents a potential advancement in targeted cancer therapy, enabling the simultaneous delivery of two distinct drugs into the same cancer cells to overcome payload resistance and enhance therapeutic efficacy. Here, we examine various methodologies for achieving site-specific dual-payload conjugation, including the use of multi-functional linkers, canonical amino acids, non-canonical amino acids, and enzyme-mediated methods, all of which facilitate precise control over payload attachment while ensuring homogeneity. We explore the implications of different conjugation techniques on drug-to-antibody ratios and the ratios of the two payloads, as well as their impact on process complexity and manufacturability. Additionally, we address the potential advantages of dual-payload ADCs compared to ADCs combined with traditional chemotherapy or single-payload ADC/ADC combinations. By evaluating these innovative methods, we aim to provide a comprehensive understanding of the current landscape in dual-payload ADC development and outline emerging directions necessary for further advancement of this promising therapeutic strategy.
Keywords: Antibody drug conjugate; conjugation; drug resistance; dual-payload; homogenous; manufacturability; next generation cancer therapy; process complexity.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




References
-
- Matsuda Y, Seki T, Yamada K, Ooba Y, Takahashi K, Fujii T, Kawaguchi S, Narita T, Nakayama A, Kitahara Y, et al. Chemical site-specific conjugation platform to improve the pharmacokinetics and therapeutic index of antibody–drug conjugates. Mol Pharm. 2021;18(11):4058–4066. doi: 10.1021/acs.molpharmaceut.1c00473. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
