p16INK4a and HPV E4 immunohistochemistry for the prediction of regression of cervical intraepithelial neoplasia grade 2-A historical cohort study
- PMID: 40323118
- PMCID: PMC12334904
- DOI: 10.1002/ijc.35469
p16INK4a and HPV E4 immunohistochemistry for the prediction of regression of cervical intraepithelial neoplasia grade 2-A historical cohort study
Abstract
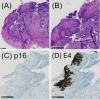
Cervical intraepithelial neoplasia grade 2 (CIN2) is a heterogeneous diagnosis with a high likelihood of spontaneous regression. Therefore, active surveillance for CIN2 has been implemented as an option in younger women in many countries. Yet, little is known about markers that may accurately predict the likelihood of regression to support active surveillance. Here, we aimed to assess whether p16INK4a and HPV E4 status are associated with the likelihood of CIN2 regression. We conducted a historical cohort study on women aged 23-40 diagnosed with untreated CIN2 following cytology-based screening. Women were diagnosed at Aarhus University Hospital, Denmark from January 2000 to December 2010. Archived tissue samples were sectioned for p16INK4a and HPV E4 immunohistochemistry and HPV genotyping. We used a modified Poisson model to estimate the relative risk of regression, adjusting for age and cytology (aRR). A total of 443 women with CIN2 were included. Most women (73.8%) were aged ≤30, and half had a high-grade cytology (48.8%). Overall, 47.6% regressed, and regression was less likely in p16INK4a-positive compared to p16INK4a-negative women (aRR 0.77; 95% CI 0.64-0.94). Among p16INK4a-positive women, the risk of regression varied by HPV type and HPVE4 status, with lower risk in HPV16-positive women compared to those without (aRR 0.54; 95% CI 0.40-0.75) and in HPVE4-negative compared to HPVE4 positive women (aRR 0.73; 95% CI 0.54-0.98). When we restricted to expert-confirmed CIN2, the risk of regression did not vary by p16INK4a or HPVE4 status, while HPV16 positive remained at a lower risk of regression compared to women without HPV16.
Keywords: HPV E4; cervical intraepithelial neoplasia; diagnostics; molecular biomarkers; p16INK4a.
© 2025 The Author(s). International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Conflict of interest statement
AH has received a consulting fee from Exeltis outside the submitted work. AH and RD have received reagents free of charge from Roche Denmark. Roche had no influence in the study design, data analyses, or the decision to publish. MS has received a consultant fee from Roche, Merck, BD Life Science, Abbott Molecular, Inovio Pharmaceuticals, and a speaker's fee from Roche and BD Life Science outside the work of this study. DJ has received funding from DDL Diagnostic for this project. MK has received grants from Cerba Research NL outside the work of this study. The remaining authors report no conflicts of interest.
Figures

References
-
- Lycke KD, Kahlert J, Damgaard RK, et al. Clinical course of cervical intraepithelial neoplasia grade 2: a population‐based cohort study. Am J Obstet Gynecol. 2023;229:656e1–e15. - PubMed
-
- Lycke KD, Petersen LK, Gravitt PE, Hammer A. Known benefits and unknown risks of active surveillance of cervical intraepithelial neoplasia grade 2. Obstet Gynecol. 2022;139:680‐686. - PubMed
-
- Damgaard RK, Jenkins D, Stoler MH, et al. Human papillomavirus genotypes and risk of persistence and progression in women undergoing active surveillance for cervical intraepithelial neoplasia grade 2. Am J Obstet Gynecol. 2024;230:655e1–e10. - PubMed
-
- Kyleback K, Ekeryd‐Andalen A, Greppe C, Bjorkenfeldt Havel C, Zhang C, Strander B. Active expectancy as alternative to treatment for cervical intraepithelial neoplasia grade 2 in women aged 25 to 30 years: ExCIN2‐a prospective clinical multicenter cohort study. Am J Obstet Gynecol. 2022;227:742e1–e11. - PubMed

