Common neuropathologic change drivers of hippocampal sclerosis of ageing
- PMID: 40323260
- PMCID: PMC12233507
- DOI: 10.1093/brain/awaf158
Common neuropathologic change drivers of hippocampal sclerosis of ageing
Abstract
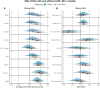
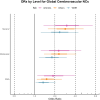
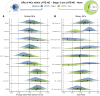
Hippocampal sclerosis of ageing (HS-A)-severe cell loss and gliosis in the hippocampal formation-is a neuropathologic change (NC) that affects up to 20% of elderly persons with dementia. The aetiology of HS-A is heterogeneous, but HS-A is strongly associated with limbic-predominant age-related TDP-43 encephalopathy NC (LATE-NC). Other NCs have also been implicated in relation to HS-A, but these associations have been inconsistent across previous studies. Also, because LATE-NC and HS-A are so strongly associated, it is important to adjust for LATE-NC when examining associations between other NCs and HS-A. The goal of this study was to examine associations of other common NCs with HS-A, both before and after adjusting for LATE-NC. We analysed the National Alzheimer's Coordinating Center (NACC) neuropathology dataset and examined associations of Alzheimer's disease NC (ADNC), Lewy bodies (LB) and cerebrovascular NCs, with HS-A, adjusting for LATE-NC in multiple ways. We used Bayesian multilevel logistic regression models with monotonic modelling for ordinal predictors and report the odds ratios (OR) or average OR across levels (aOR), along with 95% credibility intervals (CI) as well as expected frequencies of HS-A for selected models and predictor levels. Of n = 1933 autopsy participants included (average age at death of 83 years, 51.3% women), HS-A was present in 278 (14.4%). LATE-NC was strongly associated with HS-A (aOR = 3.7, 95% CI = 2.8, 5.0). While ADNC showed a modest association with HS-A in models where LATE-NC was not included as a predictor (aOR = 1.4, CI = 1.1, 1.8), this association was reduced when adjusting for LATE-NC (aOR = 1.11, CI = 0.9, 1.5); results were similar for the ADNC-related A/B/C scores and limbic LBs. However, several cerebrovascular NCs were similarly associated with HS-A both without adjusting for LATE-NC [atherosclerosis aOR = 1.4, arteriolosclerosis aOR = 1.6, white matter rarefaction (WMR) aOR = 1.4] and with adjusting for LATE-NC (atherosclerosis aOR = 1.4, arteriolosclerosis aOR = 1.5, WMR aOR = 1.3). In a combined model, LATE-NC was strongly associated with HS-A, but global cerebrovascular NCs, as well as APOE-ε4 (increased odds) and education (decreased odds), were also associated with HS-A. Predicted HS-A frequency for predictor levels of no LATE-NC or global cerebrovascular NCs was 1.5% (CI = 0.6%, 3.1%), while it was 94.5% (CI = 84%, 99.5%) for LATE-NC stage 3 and severe global cerebrovascular NC levels. LATE-NC is likely the most important cause of HS-A. While ADNC seems to be associated with HS-A through its association with LATE-NC, the association of cerebrovascular NCs with HS-A independent of LATE-NC underlines the importance of vascular factors in the aetiology of HS-A.
Keywords: amyloid; hippocampus; memory; neurodegeneration; oldest-old; tau.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures


Comment in
-
The converging paths to hippocampal sclerosis of ageing: insights from LATE-NC and vascular disease.Brain. 2025 Jul 7;148(7):2238-2239. doi: 10.1093/brain/awaf218. Brain. 2025. PMID: 40472066 No abstract available.
References
-
- Zarow C, Sitzer TE, Chui HC. Understanding hippocampal sclerosis in the elderly: Epidemiology, characterization, and diagnostic issues. Curr Neurol Neurosci Rep. 2008;8:363–370. - PubMed
-
- Dickson DW, Davies P, Bevona C, et al. Hippocampal sclerosis: A common pathological feature of dementia in very old (> or = 80 years of age) humans. Acta Neuropathol. 1994;88:212–221. - PubMed
MeSH terms
Grants and funding
- P20 AG068053/AG/NIA NIH HHS/United States
- P30 AG066515/AG/NIA NIH HHS/United States
- P30 AG062421/AG/NIA NIH HHS/United States
- P30 AG066508/AG/NIA NIH HHS/United States
- P30 AG066519/AG/NIA NIH HHS/United States
- P30 AG072973/AG/NIA NIH HHS/United States
- P30 AG066462/AG/NIA NIH HHS/United States
- P30 AG066509/AG/NIA NIH HHS/United States
- P20 AG068077/AG/NIA NIH HHS/United States
- P30 AG066546/AG/NIA NIH HHS/United States
- P30 AG072972/AG/NIA NIH HHS/United States
- P20 AG068082/AG/NIA NIH HHS/United States
- P30 AG072975/AG/NIA NIH HHS/United States
- P30 AG066444/AG/NIA NIH HHS/United States
- P30 AG066507/AG/NIA NIH HHS/United States
- P30 AG072946/AG/NIA NIH HHS/United States
- P30 AG066518/AG/NIA NIH HHS/United States
- P30 AG066511/AG/NIA NIH HHS/United States
- U24 AG072122/AG/NIA NIH HHS/United States
- P30 AG066512/AG/NIA NIH HHS/United States
- P30 AG072978/AG/NIA NIH HHS/United States
- P30 AG062429/AG/NIA NIH HHS/United States
- P30 AG062422/AG/NIA NIH HHS/United States
- R01 AG079280/AG/NIA NIH HHS/United States
- R01 AG062706/AG/NIA NIH HHS/United States
- P30 AG066530/AG/NIA NIH HHS/United States
- P30 AG072977/AG/NIA NIH HHS/United States
- NH/NIH HHS/United States
- P30 AG062677/AG/NIA NIH HHS/United States
- P20 AG068024/AG/NIA NIH HHS/United States
- P30 AG072958/AG/NIA NIH HHS/United States
- P30 AG062715/AG/NIA NIH HHS/United States
- P30 AG066506/AG/NIA NIH HHS/United States
- P30 AG066468/AG/NIA NIH HHS/United States
- P30 AG072976/AG/NIA NIH HHS/United States
- P30 AG072947/AG/NIA NIH HHS/United States
- P30 AG072931/AG/NIA NIH HHS/United States
- P30 AG066514/AG/NIA NIH HHS/United States
- P30 AG072959/AG/NIA NIH HHS/United States
- R01AG062706/AG/NIA NIH HHS/United States
- P30 AG072979/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

