Determining an appropriate fosfomycin (ZTI-01) dosing regimen in pneumonia patients by utilizing minimal PBPK modeling and target attainment analysis
- PMID: 40323339
- PMCID: PMC12135528
- DOI: 10.1128/aac.01869-24
Determining an appropriate fosfomycin (ZTI-01) dosing regimen in pneumonia patients by utilizing minimal PBPK modeling and target attainment analysis
Abstract
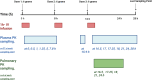
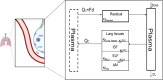
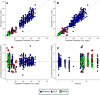
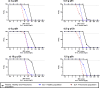
Fosfomycin, a broad-spectrum antibiotic used for uncomplicated cystitis, represents a potential promising candidate in combating resistant pneumonia. To facilitate the transition of fosfomycin to broader indications, including pneumonia, a minimal physiologically based pharmacokinetics (m-PBPK) model for fosfomycin was developed based on data from plasma, epithelial lining fluid (ELF), and alveolar macrophages (AMs) obtained from 37 healthy participants in a recently completed intrapulmonary PK study. Utilizing this mechanistic m-PBPK model enabled us to predict drug concentrations at the infection site in pneumonia patients, taking into consideration the pathophysiological changes occurring during the infection. Our prediction shows that the drug concentrations at the infection site reduced, while plasma levels remain unchanged. Monte Carlo simulations were conducted to evaluate the probability of target attainment (PTA) for various dosing regimens infused over 1 h against major hospital-acquired pneumonia pathogens in plasma and ELF. Our PTA analysis suggested that if plasma concentrations are the appropriate efficacy indicator, a dose of 4 g q8h is sufficient for Pseudomonas aeruginosa and Staphylococcus aureus infections. However, if ELF concentrations are a more accurate indicator, this dose is only effective for S. aureus pneumonia. For P. aeruginosa pneumonia, a dose of 6 g q8h is recommended, with an even higher dose of 8 g q8h necessary for pneumonia patients. In conclusion, our model provides critical insights into fosfomycin dosing for pneumonia treatment, guiding clinical study design. Furthermore, it serves as a platform to evaluate intrapulmonary pharmacokinetics for other antibiotics.
Keywords: fosfomycin; m-PBPK; pneumonia; repurposing; target attainment analysis.
Conflict of interest statement
L.G.Q. reports support from Astra-ZenecaAstraZeneca, Sanofi-Regeneron, American Lung Association, and the National Institute of Health for work unrelated to this report. E.B.W. reports institutional support from Pfizer, Moderna, Sequiris, Najit Technologies Inc., and Clinetic and support from Pfizer, Vaxcyte, ILiAD Biotechnologies, and Shionogi for work unrelated to this project. The other authors declare no conflict of interest.
Figures


References
-
- Laudy AE, Róg P, Smolińska-Król K, Ćmiel M, Słoczyńska A, Patzer J, Dzierżanowska D, Wolinowska R, Starościak B, Tyski S. 2017. Prevalence of ESBL-producing Pseudomonas aeruginosa isolates in Warsaw, Poland, detected by various phenotypic and genotypic methods. PLoS One 12:e0180121. doi: 10.1371/journal.pone.0180121 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

