Impact of the double deletion ΔG242-T243 in KPC-2 in the effectiveness of ceftazidime-avibactam and imipenem-relebactam
- PMID: 40323374
- PMCID: PMC12135508
- DOI: 10.1128/aac.01915-24
Impact of the double deletion ΔG242-T243 in KPC-2 in the effectiveness of ceftazidime-avibactam and imipenem-relebactam
Abstract
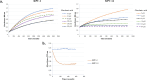
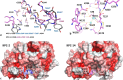
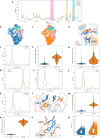
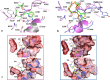
Combinations of β-lactam-diazabicyclooctane inhibitors (DBOs) like ceftazidime-avibactam (CZA) and imipenem-relebactam (IMR) have shown efficacy in treating KPC-2-producing Klebsiella pneumoniae. However, CZA-resistant K. pneumoniae strains have been identified, often linked to substitutions and/or insertions/deletions in three different loops of KPC: (i) the Ω-loop region (amino acids 164-179), (ii) the 237-243 loop; and (iii) the 266-275 loop. This study investigates the impact of the double deletion ΔG242-T243 present in KPC-14. Our results demonstrate that the lower effectiveness of CZA against KPC-14 can be explained by both increased hydrolysis of ceftazidime and a lower affinity and acylation rate by avibactam. In contrast, the IMR combination was efficient in restoring susceptibility to the KPC-14 producing-clone. Although we also observed a lower affinity and acylation rate for relebactam in KPC-14, this reduction in affinity was accompanied by a loss in the carbapenemase activity, finally resulting in an IMR susceptibility phenotype for KPC-14. Expansion of the substrate profile of KPC-14 toward ceftazidime is associated with a trade-off for carbapenems, other penicillins, and cephalosporins, as well as a higher inhibition by clavulanic acid compared to KPC-2. This study provides a better understanding of how deletions in the 237-243 loop affect the effectiveness of novel DBO-combinations and supports the hypothesis that these mutations result in CZA resistance by other different biochemical mechanisms than mutations in the Ω-loop.
Keywords: CZA; DBO; KPC-14; Klebsiella pneumoniae; avibactam; relebactam.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Papp-Wallace KM, Barnes MD, Alsop J, Taracila MA, Bethel CR, Becka SA, van Duin D, Kreiswirth BN, Kaye KS, Bonomo RA. 2018. Relebactam is a potent inhibitor of the KPC-2 β-lactamase and restores imipenem susceptibility in KPC-producing Enterobacteriaceae. Antimicrob Agents Chemother 62:9. doi:10.1128/AAC.00174-18 - DOI - PMC - PubMed
-
- Sader HS, Mendes RE, Duncan L, Kimbrough JH, Carvalhaes CG, Castanheira M. 2023. Ceftazidime-avibactam, meropenem-vaborbactam, and imipenem-relebactam activities against multidrug-resistant Enterobacterales from United States Medical Centers (2018-2022). Diagn Microbiol Infect Dis 106:115945. doi:10.1016/j.diagmicrobio.2023.115945 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

