Web-Based Cancer Symptom Self-Management System: A Randomized Clinical Trial
- PMID: 40323601
- PMCID: PMC12053558
- DOI: 10.1001/jamanetworkopen.2025.8353
Web-Based Cancer Symptom Self-Management System: A Randomized Clinical Trial
Abstract
Importance: Patients with cancer and cancer survivors frequently experience symptoms that increase the need for health care services and impair quality of life. Effective symptom management is critical for comprehensive patient-centered cancer care.
Objective: To evaluate the effectiveness of adding a bilingual (English and Spanish), web-based self-management program to an electronic health record (EHR)-integrated patient-reported outcome for cancer (cPRO) assessment in reducing symptom burden and health care resource use (HCRU).
Design, setting, and participants: This patient-level randomized clinical trial was performed at the Northwestern Memorial HealthCare system in Chicago, Illinois. Participants included 1614 adult patients with cancer or cancer survivors in 30 clinics who were enrolled between April 1, 2020, and April 8, 2023, and followed up for 12 months until May 8, 2024.
Interventions: Usual care (UC) consisting of an EHR-integrated cPRO assessment or enhanced care (EC), which offered an additional tailored web-based self-management program.
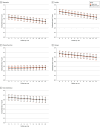
Main outcomes and measures: Patient-Reported Outcomes Measurement Information System measures of anxiety, depression, fatigue, pain interference, and physical function collected at baseline and monthly for 12 months. Secondary outcomes included HCRU measures (inpatient and/or observation visits and days, emergency department and/or urgent care visits, and days of hospital stay).
Results: A total of 1614 patients were included in the analysis, with 804 randomized to EC and 810 to UC. The mean (SD) age was 61 (13) years; 1095 patients (67.8%) were female. Only 419 EC participants (52.1%) accessed the website, with only 197 (47%) returning; the median time per visit was 45 seconds (IQR, 45-105 seconds). There were no statistically significant differences between EC and UC across the cPRO outcomes over 12 months. The mean change from baseline at each assessment time point for treatment effects (EC vs UC) ranged from -0.19 (95% CI, -0.86 to 0.33; P = .64) for physical function to 0.11 (95% CI, -0.75 to 0.79; P = .87) for fatigue. Zero-inflated negative binomial and logistic regression models showed no significant differences in HCRU outcomes: inpatient and/or observation visits (incidence rate ratio [IRR], 0.90; 95% CI, 0.72-1.12), emergency department and/or urgent care visits (IRR, 0.99; 95% CI, 0.84-1.16), and days of hospital stay (IRR, 1.05; 95% CI, 0.83-1.33).
Conclusions and relevance: In this randomized clinical trial, adding a bilingual web-based self-management program to EHR-integrated cPRO did not reduce symptom burden or HCRU compared with cPRO alone. Low engagement with the web-based program highlights the need for strategies to enhance engagement and tailor interventions to those who would benefit most.
Trial registration: ClinicalTrials.gov Identifier: NCT03988543.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

