Midurethral Sling vs OnabotulinumtoxinA in Females With Urinary Incontinence: The MUSA Randomized Clinical Trial
- PMID: 40323617
- PMCID: PMC12053799
- DOI: 10.1001/jama.2025.4682
Midurethral Sling vs OnabotulinumtoxinA in Females With Urinary Incontinence: The MUSA Randomized Clinical Trial
Abstract
Importance: Mixed urinary incontinence, which includes both stress and urgency urinary incontinence, adversely affects quality of life and can be difficult to manage. Studies comparing procedural-based treatments for mixed urinary incontinence are lacking.
Objective: To determine whether intradetrusor onabotulinumtoxinA is more effective than midurethral sling for the treatment of mixed urinary incontinence in females.
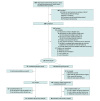
Design, setting, and participants: Randomized, superiority trial involving females (aged ≥21 years) with moderate to severe bother from both stress and urgency urinary incontinence who had unsuccessful conservative treatments and oral medications. The study was conducted at 7 US sites with enrollment between July 2020 and September 2022; the last date of follow-up was December 29, 2023.
Interventions: Intradetrusor injection of onabotulinumtoxinA, 100 U (treatment focused on the urgency component), vs surgical synthetic mesh midurethral sling (treatment focused on the stress component). Recipients of onabotulinumtoxinA could receive an additional injection between 3 and 6 months. All participants could receive additional treatment (including crossover to the alternative treatment) between 6 and 12 months.
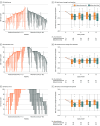
Main outcomes and measures: The primary outcome was change at 6 months in mixed incontinence symptoms as measured by the Urogenital Distress Inventory (UDI) total score (0-300 points; higher scores indicate worse symptoms; minimal clinically important difference, 26.1). Secondary outcomes included stress and irritative UDI subscores.
Results: Among 150 females randomized, 137 were treated, had postbaseline outcome data, and were included in the primary analysis (mean [SD] age, 59.0 [11.5] years). Both groups demonstrated mean improvement in UDI total score at 6 months with no significant difference between groups (onabotulinumtoxinA: -66.8 points [95% CI, -84.9 to -48.8]; sling: -84.9 [95% CI, -100.5 to -69.3]; mean difference, 18.1 points [95% CI, -4.6 to 40.7]; P = .12). For secondary outcomes, greater UDI stress score improvement was seen with the sling (-45.2 [95% CI, -53.7 to -36.8]) compared with onabotulinumtoxinA (-25.1 [95% CI, -34.1 to -16.1]) (P < .001); however, no significant difference was seen between groups in UDI irritative score (onabotulinumtoxinA: -32.9 [95% CI, -40.3 to -25.6] vs sling: -27.4 [95% CI, -34.6 to -20.3]; P = .27). In the onabotulinumtoxinA group, 12.7% and 28.2% received a second injection by 6 and 12 months, respectively. By 12 months, 30.3% in the sling group received onabotulinumtoxinA, and 15.5% in the onabotulinumtoxinA group received a sling. Overall, adverse events were not different between groups.
Conclusions and relevance: There was no observed difference in UDI total score improvement at 6 months between the onabotulinumtoxinA and midurethral sling groups in females with moderate to severe mixed urinary incontinence who previously did not respond to conservative treatments. These findings may help inform treatment decisions based on patient preference in partnership with clinician recommendations.
Trial registration: ClinicalTrials.gov Identifier: NCT04171531.
Conflict of interest statement
Figures
Comment in
-
Balancing Midurethral Sling vs OnabotulinumtoxinA for Mixed Urinary Incontinence.JAMA. 2025 Jun 3;333(21):1872-1873. doi: 10.1001/jama.2025.6209. JAMA. 2025. PMID: 40323621 No abstract available.
References
-
- Haylen BT, de Ridder D, Freeman RM, et al. ; International Urogynecological Association; International Continence Society . An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(1):4-20. doi: 10.1002/nau.20798 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

