Itaconate potentiates hepatic gluconeogenesis through NRF2 induction
- PMID: 40323920
- PMCID: PMC12052187
- DOI: 10.1371/journal.pone.0322946
Itaconate potentiates hepatic gluconeogenesis through NRF2 induction
Abstract
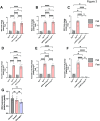
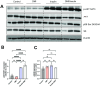
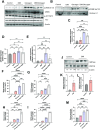
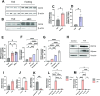
The interplay between systemic metabolism and immune responses is increasingly recognized as a significant factor in the dysregulation of glucose homeostasis associated with diabetes and obesity. Immune metabolites play crucial roles in mediating this crosstalk, with itaconate emerging as an important immune metabolite involved in the inflammatory response of macrophages. Recent studies have highlighted the role of itaconate as a regulator of glucose metabolism, particularly in the context of obesity, although the underlying mechanisms remain poorly understood. In this study, we identified itaconate as one of the metabolites that significantly increase in the liver during fasting compared to fed conditions. Mechanistically, we found that itaconate enhances glucagon-induced liver gluconeogenesis independently of insulin signaling. Notably, itaconate upregulates the expression of gluconeogenic genes both under basal conditions and in the presence of palmitic acid. Furthermore, our data indicate that the effects of itaconate occur independently of CREB activation. Instead, we demonstrate that these potentiating effects are mediated through the induction of nuclear factor erythroid 2-related factor 2 (NRF2). Our findings demonstrate that itaconate has a glucagon-potentiating effects in the liver, suggesting that itaconate may play a significant role in the pathogenesis of metabolic-associated liver diseases.
Copyright: © 2025 El-Derany et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

