A novel mouse model of hemoglobin SC disease reveals mechanisms underlying beneficial effects of hydroxyurea
- PMID: 40324066
- PMCID: PMC12290519
- DOI: 10.1182/blood.2024028136
A novel mouse model of hemoglobin SC disease reveals mechanisms underlying beneficial effects of hydroxyurea
Abstract
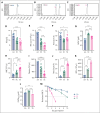
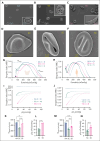
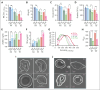
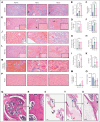
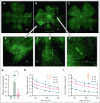
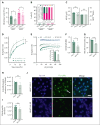
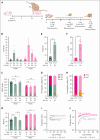
Sickle cell hemoglobin C (HbSC) disease results from compound heterozygosity of hemoglobin S (HbS) and hemoglobin C (HbC), comprising 30% of sickle cell disease (SCD). HbC induces red blood cell (RBC) dehydration/xerocytosis, which promotes sickling. HbSC-SCD causes significant morbidity despite being milder than homozygous HbSS-SCD. Current research/treatment strategies have focused on HbSS-SCD, whereas patients with HbSC are deprived of disease-modifying/transformative therapies because of lack of preclinical models. We generated HbSC mice, which resemble human HbSC-SCD: HbSC erythrocytes showed marked xerocytosis. Anemia, hemolysis, inflammation, and organ damage were milder than HbSS mice but hypoxia/reperfusion injury was similar. Retinopathy developed at higher frequency than HbSS mice (66.7% vs 16.7%; P < .05), as in patients with HbSC-SCD. Although HbSC RBCs sickled at lower oxygen tension than HbSS RBCs, they did not completely recover deformability after hypoxia/reoxygenation. Using the HbSC mice, we studied the mechanism by which hydroxyurea causes significant clinical benefit in patients with HbSC-SCD, despite minimal/modest increases in fetal Hb (HbF). We found hydroxyurea had distinct non-HbF and HbF effects. Hydroxyurea did not increase HbF in adult HbSC/HbSS mice but reduced RBC reactive oxygen species, ferryl Hb, and Heinz-body formation, thereby reducing membrane damage; however, RBC hydration was unaffected. When given to unborn pups before γ-globin expression was switched off, and continued postnatally, we could induce HbF in both HbSC and HbSS mice (higher HbF in HbSS vs HbSC mice). Minimal increases in HbF (∼1%) improved HbSC RBC hydration. Peak HbF levels of 7% in HbSC mice abrogated sickling. Overall, this HbSC model will help bridge the knowledge gap in mechanistic/therapeutic studies in this neglected disease.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

Comment in
-
HbSC gets its mouse model 75 years after discovery.Blood. 2025 Jul 3;146(1):1-2. doi: 10.1182/blood.2025029523. Blood. 2025. PMID: 40608353 No abstract available.
References
-
- Serjeant GR, Vichinsky E. Variability of homozygous sickle cell disease: the role of alpha and beta globin chain variation and other factors. Blood Cells Mol Dis. 2018;70:66–77. - PubMed
-
- Segbefia C, Luchtman-Jones L, Seeing haemoglobin SC. Challenging the misperceptions. Br J Haematol. 2024;205(2):404–405. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

