Exploiting thioether reactivity to label mycobacterial glycans
- PMID: 40324093
- PMCID: PMC12088412
- DOI: 10.1073/pnas.2422185122
Exploiting thioether reactivity to label mycobacterial glycans
Abstract
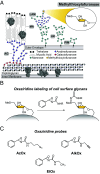
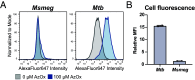
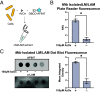
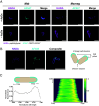
Mycobacterium tuberculosis (Mtb) is a leading cause of death worldwide. Mtb cell envelope glycans are potent virulence factors that play key roles in mediating infection of host tissues and modulating the host immune response. However, there are few ways to site-selectively modify and label these or any glycans to study their functions in disease. This gap arises because glycans generally lack functional groups amenable to bioconjugation strategies. Methylthioxylofuranose (MTX), a rare monosaccharide in select pathogenic mycobacteria, is an exception. MTX is appended to mannose-capped lipoarabinomannan (ManLAM), an antigenic glycolipid in the Mtb cell envelope implicated in downregulating the host immune system during infection. MTX is unique not only in its prevalence but also in its functionality-it contains a thioether not present in other glycans. We envisioned exploiting the MTX thioether to selectively label ManLAM with an oxaziridine probe. Here, we show that MTX-containing glycans can be labeled selectively in the test tube and live cells, highlighting the reactivity and accessibility of this motif. Our approach labels ManLAM efficiently despite the presence of protein methionine residues and can distinguish between different mycobacterial species. Using an oxaziridine equipped with a reporter, we could visualize ManLAM localization in live cells and a macrophage infection model, highlighting the stability of the label and the cell envelope in this environment. These studies will enable investigations of dynamic changes in a critical Mtb cell envelope component during infection. Moreover, the selective reactivity of thioethers can be leveraged to expand the repertoire of glycan bioconjugation strategies.
Keywords: Mycobacterium tuberculosis; bioconjugation; glycans; mannose-capped lipoarabinomannan; mycobacteria.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

References
-
- Global Tuberculosis Report 2022 (World Health Organization, ed. 1, 2022).
-
- Pai M., et al. , Tuberculosis. Nat. Rev. Dis. Primers 2, 1–23 (2016).
-
- Colditz G. A., et al. , Efficacy of BCG vaccine in the prevention of tuberculosis: Meta-analysis of the published literature. JAMA 271, 698–702 (1994). - PubMed
-
- Dulberger C. L., Rubin E. J., Boutte C. C., The mycobacterial cell envelope—a moving target. Nat. Rev. Microbiol. 18, 47–59 (2020). - PubMed
MeSH terms
Substances
Grants and funding
- U19 AI162584/AI/NIAID NIH HHS/United States
- R01A1022553/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- P30 CA014051/CA/NCI NIH HHS/United States
- R01 AR073252/AR/NIAMS NIH HHS/United States
- R01 AI165573/AI/NIAID NIH HHS/United States
- R01 AI126592/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- GRFP 1745302/NSF | EDU | Division of Graduate Education (DGE)
- R1AR073252/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- Al-126592/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
LinkOut - more resources
Full Text Sources
Miscellaneous

