Characteristics of Patients and Prognostic Factors Across Treatment Lines in Metastatic Colorectal Cancer: An Analysis From the Aide et Recherche en Cancérologie Digestive Database
- PMID: 40324123
- PMCID: PMC12169864
- DOI: 10.1200/JCO-24-01968
Characteristics of Patients and Prognostic Factors Across Treatment Lines in Metastatic Colorectal Cancer: An Analysis From the Aide et Recherche en Cancérologie Digestive Database
Abstract
Purpose: Several lines of treatment can be used sequentially in patients with metastatic colorectal cancer. We investigated the evolution of patient/tumor characteristics and their prognostic impact across treatment lines to develop an overall prognostic score (OPS).
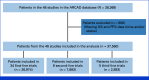
Patients and methods: Individual patient data from 48 randomized trials were analyzed. The end point was overall survival (from random assignment to death). Missing data were imputed. The complete data set was then separated into construction (80%) and validation sets (20%). The Cox's model was used to define risk groups for survival using the OPS. The discrimination capability was assessed in each treatment-line via bootstrapping to obtain optimism-corrected calibration and discrimination C-indices. Internal validation was done in the validation set.
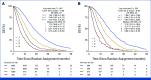
Results: A total of 37,560 patients (26,974 in first-line [1L], 7,693 in second-line [2L], and 2,893 in third-line [3L]) were analyzed. Some clinical, biological, and molecular characteristics of patients/tumors included in therapeutic trials evolve over the lines. Seven independent prognostic variables were retained in the final multivariate model common to all lines: Eastern Cooperative Oncology Group performance status, hemoglobin, platelet count, WBC/absolute neutrophil count ratio, lactate dehydrogenase, alkaline phosphatase, and the number of metastatic sites. The OPS was used to define four patient subgroups with significantly different prognoses in 1L, 2L, and 3L, separately, with adequate C-indices: 0.65, 0.66, and 0.69 in the construction set and 0.65, 0.66, and 0.68 in the validation set, respectively. The OPS was not predictive, with 3L drugs (v placebo) or subsequent line (2L/1L or 3L/2L) extending survival in all prognostic groups.
Conclusion: The same prognostic model using practical variables can be used before all treatment lines. The OPS could better stratify patients in future clinical trials and help to therapeutic decision in routine practice.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures



References
-
- Cremolini C, Antoniotti C, Stein A, et al. Individual patient data meta-analysis of FOLFOXIRI plus bevacizumab versus doublets plus bevacizumab as initial therapy of unresectable metastatic colorectal cancer J Clin Oncol 38:3314–3324.2020 - PubMed
-
- Cervantes A, Adam R, Roselló S, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:10–32. - PubMed
-
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. - PubMed
-
- Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

