SEARCH Study: Text Messages and Automated Phone Reminders for HPV Vaccination in Uganda: Randomized Controlled Trial
- PMID: 40324213
- PMCID: PMC12068829
- DOI: 10.2196/63527
SEARCH Study: Text Messages and Automated Phone Reminders for HPV Vaccination in Uganda: Randomized Controlled Trial
Abstract
Background: Cervical cancer is currently the leading female cancer in Uganda. Most women are diagnosed with late-stage disease. Human papillomavirus (HPV) vaccination is the single most important primary preventive measure. While research regarding text message vaccine reminder use is strong in the United States, their use has not yet been demonstrated in a preteen and adolescent population in subSaharan Africa or other low- and middle-income countries.
Objective: The objective of this pilot randomized controlled trial was to assess the impact of vaccine reminders with embedded interactive educational information on timeliness of HPV vaccination in Kampala, Uganda.
Methods: In this randomized controlled trial conducted in 2022, caregivers of adolescents needing a first or second HPV vaccine dose were recruited from an adolescent clinic and three community health centres in Kampala, Uganda. Families (n=154) were randomized 1:1 into intervention versus usual care, stratified by dose (ie, initiation, completion) and language (ie, English, Luganda) within each site. Intervention caregivers received a series of automated, personalized text messages or automated phone calls based on family preference. Five messages were sent before the due date, including both static and interactive educational information, with five follow-up messages for those unvaccinated. Receipt of the needed dose by 24 weeks postenrollment was assessed by χ2, regression, and Kaplan-Meier with log-rank test. All analyses were conducted using intention-to-treat principles.
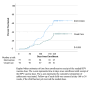
Results: Overall, 154 caregivers were enrolled (51.3% for dose 1; 48.7% for dose 2) and 64.3% (n=99) spoke Luganda. Among individuals in the intervention arm, 62% (48/78) requested SMS text message reminders and 38% (n=30) requested automated phone reminders. There was no significant difference in requested mode by HPV vaccine dose or language. Intervention adolescents were more likely to receive the needed dose by 24 weeks (51/78, 65.4% vs 27/76, 35.5%; P<.001; RR 1.8; 95% CI 1.3-2.6). There was no interaction by dose or language. There was no difference in vaccination between those requesting SMS text message versus phone reminders (32/49, 65.3% vs 19/30, 63.3%; P=.86). The number needed to message for one additional vaccination was 3.4 (95% CI 2.2-6.8). Kaplan-Meier curves demonstrated more timely vaccination in the intervention arm (P<.001).
Conclusions: In this novel trial, SMS text message and automated phone reminders were effective in promoting more timely HPV vaccination in this population.
Trial registration: ClinicalTrials.gov NCT05151367.
Keywords: chi square; Africa; HPV; RCT; SMS; Uganda; adolescent; adolescent medicine; caregiver; global health; human papillomavirus; low income country; mHealth; middle income country; mobile health; randomized controlled trial; regression; subSaharan Africa; teen; teenager; text messaging; vaccination; vaccine; youth.
© Sabrina B Kitaka, Joseph Rujumba, Sarah K Zalwango, Betsy Pfeffer, Lubega Kizza, Juliane P Nattimba, Ashley B Stephens, Nicolette Nabukeera-Barungi, Chelsea S Wynn, Juliet N Babirye, John Mukisa, Ezekiel Mupere, Melissa S Stockwell. Originally published in JMIR mHealth and uHealth (https://mhealth.jmir.org).
Conflict of interest statement
Figures
References
-
- Human papillomavirus and cancer. World Health Organization. 2024. [10-09-2023]. https://www.who.int/news-room/fact-sheets/detail/human-papilloma-virus-a... URL. Accessed.
-
- Bruni L, Albero G, Serrano B, et al. ICO/IARC information centre on HPV and cancer (HPV information centre); 2023. [10-09-2023]. Human papillomavirus and related diseases in the world. summary report.https://hpvcentre.net/statistics/reports/XWX.pdf URL. Accessed.
-
- Shaping a strategy to introduce HPV vaccines in uganda-formative research results from the HPV vaccines: evidence for impact project. PATH and Child Health and Development Centre (CHDC) 2009. [10-09-2023]. https://screening.iarc.fr/doc/PATH_FRTS_Uganda.pdf URL. Accessed.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical