The prognostic value of halp score in predicting the efficacy of nivolumab treatment in metastatic malignant melanoma patients: A real-life, retrospective, single center analysis
- PMID: 40324274
- PMCID: PMC12055156
- DOI: 10.1097/MD.0000000000042261
The prognostic value of halp score in predicting the efficacy of nivolumab treatment in metastatic malignant melanoma patients: A real-life, retrospective, single center analysis
Abstract
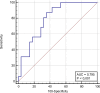
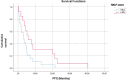
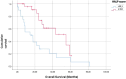
Patients with metastatic malignant melanoma have a survival rate of less than one year. Nivolumab, a monoclonal antibody against programmed cell death 1 (PD-1) receptor, has improved survival in patients without BRAF mutations. The HALP score, calculated from hemoglobin, albumin, lymphocyte, and platelet levels, provides information about a patient immune and nutritional status. High HALP scores have been associated with a better prognosis in various cancers. This study aimed to investigate the effect of high HALP scores on response to nivolumab treatment in patients with metastatic malignant melanoma. A retrospective study was conducted on 44 patients with metastatic malignant melanoma treated with nivolumab at Adana City Training and Research Hospital between 2014 and 2021. Patients who received dabrafenib-trametinib before nivolumab treatment were excluded. The HALP scores were calculated using laboratory parameters before the first nivolumab treatment. Statistical analyses were performed using SPSS version 25.0. The study included 22 female and 22 male patients with a mean age of 61.4 ± 15.6 years. Of the patients, 10 (27.2%) had a positive BRAF mutation, whereas 34 (77.3%) did not. The HALP score cutoff value was determined as 30.1. Patients with high HALP scores had significantly longer progression-free survival (PFS) and overall survival (OS) compared to those with low HALP scores (PFS: median 5.8 vs 3.1 months, P = .041; OS: median 54.9 vs 14.4 months, P = .005). In this study, we found that high HALP scores were significantly associated with longer PFS and OS in metastatic malignant melanoma patients receiving nivolumab treatment. HALP score was associated with both PFS and OS in patients with metastatic malignant melanoma treated with nivolumab. This immuno-nutritional parameter may be useful in various cancers; however, further prospective studies with larger patient cohorts are needed for clinical application.
Keywords: HALP score; immuno-nutritional parameter; metastatic malignant melanoma; nivolumab; prognosis.
Copyright © 2025 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no funding and conflicts of interest to disclose.
Figures
References
-
- Welch HG, Mazer BL, Adamson AS. The rapid rise in cutaneous melanoma diagnoses. N Engl J Med. 2021;384:72–9. - PubMed
-
- Bolick NL, Geller AC. Epidemiology and screening for melanoma. Hematol Oncol Clin North Am. 2024;38:889–906. - PubMed
-
- Ascierto PA, Del Vecchio M, Mandalá M, et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB–C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21:1465–77. - PubMed
-
- Dummer R, Hauschild A, Santinami M, et al. Five-year analysis of adjuvant dabrafenib plus trametinib in stage III Melanoma. N Engl J Med. 2020;383:1139–48. - PubMed
-
- Long GV, Swetter SM, Menzies AM, Gershenwald JE, Scolyer RA. Cutaneous melanoma. Lancet (London, England). 2023;402:485–502. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

