Oxidative stress response and NRF2 signaling pathway in autism spectrum disorder
- PMID: 40324316
- PMCID: PMC12099462
- DOI: 10.1016/j.redox.2025.103661
Oxidative stress response and NRF2 signaling pathway in autism spectrum disorder
Abstract
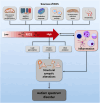
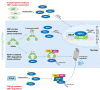
The prevalence of autism spectrum disorder (ASD), a neurodevelopmental disorder characterized by impairments in social communication and restricted/repetitive behavioral patterns, has increased significantly over the past few decades. The etiology of ASD involves a highly complex interplay of genetic, neurobiological, and environmental factors, contributing to significant heterogeneity in its clinical phenotype. In the evolving landscape of ASD research, increasing evidence suggests that oxidative stress, resulting from both intrinsic and extrinsic factors, may be a crucial pathophysiological driver in ASD, influencing neurodevelopmental processes that underlie behavioral abnormalities. Elevated levels of oxidative stress biomarkers, including lipid peroxides, protein oxidation products, and DNA damage markers, alongside deficient antioxidant enzyme activity, have been consistently linked to ASD. This may be attributed to dysregulated activity of nuclear factor erythroid 2-related factor 2 (NRF2), a pivotal transcription factor that maintains cellular redox homeostasis by orchestrating the expression of genes involved in antioxidant defenses. Here, we summarize the converging evidence that redox imbalance in ASD may result from NRF2 dysregulation, leading to reduced expression of its target genes. We also highlight the most promising antioxidant compounds under investigation, which may restore NRF2 activity and ameliorate ASD behavioral symptoms.
Keywords: Antioxidants; Autism; Inflammation; NRF2; Oxidative stress.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Figures
References
-
- Asif M., Martiniano H.F.M.C., Marques A.R., Santos J.X., Vilela J., Rasga C., Oliveira G., Couto F.M., Vicente A.M. Identification of biological mechanisms underlying a multidimensional ASD phenotype using machine learning. Transl. Psychiatry. 2020;10:1–12. doi: 10.1038/s41398-020-0721-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

