New platinum derivatives selectively cause double-strand DNA breaks and death in naïve and cisplatin-resistant cholangiocarcinomas
- PMID: 40324694
- PMCID: PMC12547501
- DOI: 10.1016/j.jhep.2025.04.034
New platinum derivatives selectively cause double-strand DNA breaks and death in naïve and cisplatin-resistant cholangiocarcinomas
Abstract
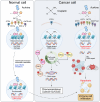
Background & aims: Patients with cholangiocarcinoma (CCA) have poor prognosis. Current cisplatin-based first-line chemotherapy offers limited survival benefit. Cisplatin induces single-strand DNA breaks, activating DNA repair mechanisms that diminish its effectiveness. Here, we present the design, chemical synthesis, and therapeutic evaluation of a new generation of chemotherapeutic agents (Aurkines) with unique polyelectrophilic properties. These agents cause a high frequency of double-strand DNA breaks, bypassing DNA repair, and promoting cancer cell death.
Methods: Two novel compounds, Aurkine 16 and Aurkine 18, were designed and evaluated for their antitumor effects in both naïve and cisplatin-resistant CCA cells, cancer-associated fibroblasts, healthy cholangiocytes, and in vivo models.
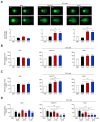
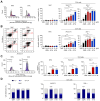
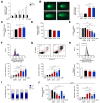
Results: Aurkines effectively induced double-strand DNA breaks, leading to increased DNA damage and elevated levels of reactive oxygen species, resulting in greater cytotoxicity than cisplatin in CCA cells. Phosphoproteomic and molecular analysis revealed that cisplatin activates DNA repair pathways, while Aurkines primarily induce apoptosis. Importantly, Aurkines also triggered apoptosis in cisplatin-resistant CCA cells and cancer-associated fibroblasts without harming healthy cholangiocytes. Additionally, Aurkines demonstrated cytotoxicity in other cisplatin-resistant cancers, such as breast and ovarian cancer. This tumor selectivity results from reduced uptake, increased efflux, and compact chromatin structure in normal cells, limiting Aurkine-DNA interactions. In vivo, Aurkines inhibited the growth of subcutaneous naïve and cisplatin-resistant CCA tumors, as well as orthotopic tumors in immunocompetent mice, promoting antitumor immune cell recruitment without any adverse events. Transport studies revealed that Aurkines were selectively taken up by OCT1, OCT3, CTR1, and OATP1A2, whereas only CTR1 transported cisplatin.
Conclusions: Aurkines represent promising therapeutic drugs for both naïve and cisplatin-resistant cancers due to their unique polyelectrophilic properties and selective targeting of malignant cells.
Impact and implications: This study introduces a novel therapeutic strategy designed to induce frequent double-strand DNA breaks selectively in both naïve and cisplatin-resistant cancer cells, without evident toxic side effects at therapeutic doses. This approach may form the basis for new strategies to overcome the critical challenge of drug resistance in cancer treatment and has the potential to be a breakthrough not only for the treatment of biliary tumors but also for other cancers.
Keywords: Cancer; Chemoresistance; Chemotherapy; DNA damage.
Copyright © 2025 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest FPC is scientific advisor of Quimatryx Ltd (quimatryx.com). Remaining authors have no conflicts of interest to declare related to this manuscript. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures




References
-
- Izquierdo-Sanchez L., Lamarca A., La Casta A., et al. Cholangiocarcinoma landscape in Europe: diagnostic, prognostic and therapeutic insights from the ENSCCA Registry. J Hepatol. 2022;76:1109–1121. - PubMed
-
- Valle J., Wasan H., Palmer D.H., et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. - PubMed
-
- Oh D.-Y., Ruth He A., Qin S., et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022;1:1–11. - PubMed
-
- Kelley R.K., Ueno M., Yoo C., et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. 2023;401:1853–1865. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

