Oscillatory brain dynamics underlying affective face processing
- PMID: 40324903
- PMCID: PMC12094162
- DOI: 10.1093/scan/nsaf047
Oscillatory brain dynamics underlying affective face processing
Abstract
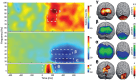
Facial expressions are ubiquitous and highly reliable social cues. Decades of research has shown that affective faces undergo facilitated processing across a distributed brain network. However, few studies have examined the multispectral brain dynamics underlying affective face processing, which is surprising given the multiple brain regions and rapid temporal dynamics thought to be involved. Herein, we used magnetoencephalography to derive dynamic functional maps of angry, neutral, and happy face processing in healthy adults. We found stronger theta oscillations shortly after the onset of affective relative to neutral faces (0-250 ms), within distributed ventral visual and parietal cortices, and the anterior hippocampus. Early gamma oscillations (100-275 ms) were strongest for angry faces in the inferior parietal lobule, temporoparietal junction, and presupplementary motor cortex. Finally, beta oscillations (175-575 ms) were stronger for neutral relative to affective expressions in the middle occipital and fusiform cortex. These results are consistent with the literature in regard to the critical brain regions, and delineate a distributed network where multispectral oscillatory dynamics support affective face processing through the rapid merging of low-level visual inputs to interpret the emotional meaning of each facial expression.
Keywords: MEG; affective faces; beta desynchronization; gamma activity; magnetoencephalography; theta activity.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
None declared.
Figures



References
-
- Aftanas LI, Varlamov AA, Pavlov SV et al. Time-dependent cortical asymmetries induced by emotional arousal: EEG analysis of event-related synchronization and desynchronization in individually defined frequency bands. Int J Psychophysiol 2002;44:67–82. doi: 10.1016/S0167-8760(01)00194-5 - DOI - PubMed
-
- Amaral DG. The amygdala and social behavior: what’s fear got to do with it?. In: Gorman JM (ed.), Fear and Anxiety: the Benefits of Translational Research. Arlington, VA, US: American Psychiatric Publishing Inc, 2004;251–63.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

